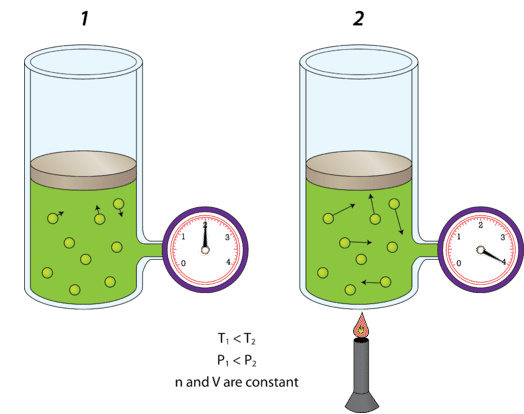
CIE IGCSE Physics (0625) Particle model Study Notes - New Syllabus
CIE IGCSE Physics (0625) Particle model Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of Particle model
Key Concepts:
- Particle Model of Matter
- Particle Motion and Temperature
- Gas Pressure and Particle Motion
- Brownian Motion and the Kinetic Particle Model
Particle Model of Matter
States of Matter: Solids, Liquids, Gases
- All substances are made of particles – atoms, molecules, or ions – that behave differently in each state.
- The properties of a substance in each state depend on:
- How the particles are arranged
- How far apart they are (separation)
- How they move (motion)
- The strength of the forces between them
Solids
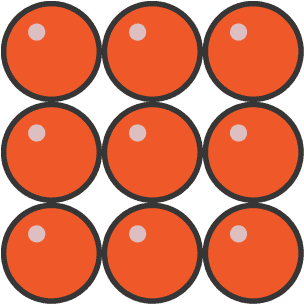
- Arrangement: Particles are packed tightly in a regular, fixed structure.
- Separation: Very little space between particles – they are very close together.
- Motion: Particles vibrate about fixed positions; they cannot move freely.
- Forces: Strong attractive forces hold particles in place.
- Properties: Solids have a fixed shape and volume, cannot flow, and are not easily compressed.
Liquids
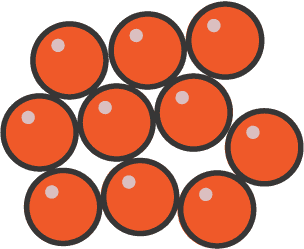
- Arrangement: Particles are close together but not in a regular pattern.
- Separation: Small gaps between particles, slightly further apart than in solids.
- Motion: Particles move and slide past each other.
- Forces: Weaker forces than solids, but still significant.
- Properties: Liquids have a fixed volume but can flow and take the shape of their container.
Gases

- Arrangement: Particles are randomly arranged and spread far apart.
- Separation: Large distances between particles – much greater than in solids and liquids.
- Motion: Particles move quickly and randomly in all directions.
- Forces: Very weak attractive forces – particles move independently.
- Properties: Gases have no fixed shape or volume, can be compressed, and expand to fill their container.
Effect of Particle Forces and Distances on Properties

The strength of inter-particle forces and the distance between particles directly control how a substance behaves in each state. These two factors affect:
- Shape and volume of the substance
- Ability to flow or be poured
- Compressibility (how much it can be squeezed into a smaller volume)
- Diffusion rate (how fast particles spread out)
Detailed Comparison:
| State | Forces Between Particles | Distance Between Particles | Resulting Properties |
|---|---|---|---|
| Solid | Very strong – particles held tightly in place | Very close – no gaps | Fixed shape and volume Cannot be compressed Cannot flow |
| Liquid | Medium – enough to hold particles close but allow movement | Close – small gaps between particles | Fixed volume, no fixed shape Can flow Hard to compress |
| Gas | Very weak – particles move freely | Very far apart – lots of empty space | No fixed shape or volume Can flow easily Easily compressed Fast diffusion |
Example:
Compare the particle arrangement and motion in a solid and a gas. Explain how these differences lead to different properties.
▶️ Answer/Explanation
Solids:
Particles are tightly packed in a regular structure. They vibrate in fixed positions but do not move freely. The strong forces between particles keep the shape and volume fixed.
Gases:
Particles are far apart and move quickly in random directions. Weak forces mean gases have no fixed shape or volume and can be compressed easily.
Conclusion:
The stronger forces and close arrangement in solids explain their rigidity, while the weaker forces and large separations in gases allow them to expand and flow freely.
Example:
Explain why a gas can be compressed easily but a solid cannot, in terms of particle spacing and forces.
▶️ Answer/Explanation
In a gas, particles are very far apart with a lot of empty space between them. The weak intermolecular forces allow particles to be pushed closer together easily when compressed.
In a solid, particles are already tightly packed with no significant empty space. The strong forces between them resist any attempt to compress, making solids incompressible.
Example:
Two containers of equal volume are filled with the same number of particles of the same substance: one in the liquid state and one in the gas state. Without changing the number of particles, explain why the gas can be compressed but the liquid cannot.
▶️ Answer/Explanation
Compressibility depends on how much empty space exists between particles.
Explanation:
In a gas, particles are far apart and move freely, leaving large gaps between them. These gaps allow the gas to be compressed, as particles can be pushed closer together.
In a liquid, particles are already close together with only tiny gaps. There is almost no empty space to eliminate, so compressing a liquid is extremely difficult.
Conclusion:
The large inter-particle spaces in a gas allow compression, whereas the closely packed particles in a liquid resist it.
\( \boxed{\text{Gases are compressible due to large spaces between particles; liquids are not.}} \)
Particle Motion and Temperature
Particle Motion and Temperature
The temperature of a substance is directly related to the average kinetic energy of its particles.
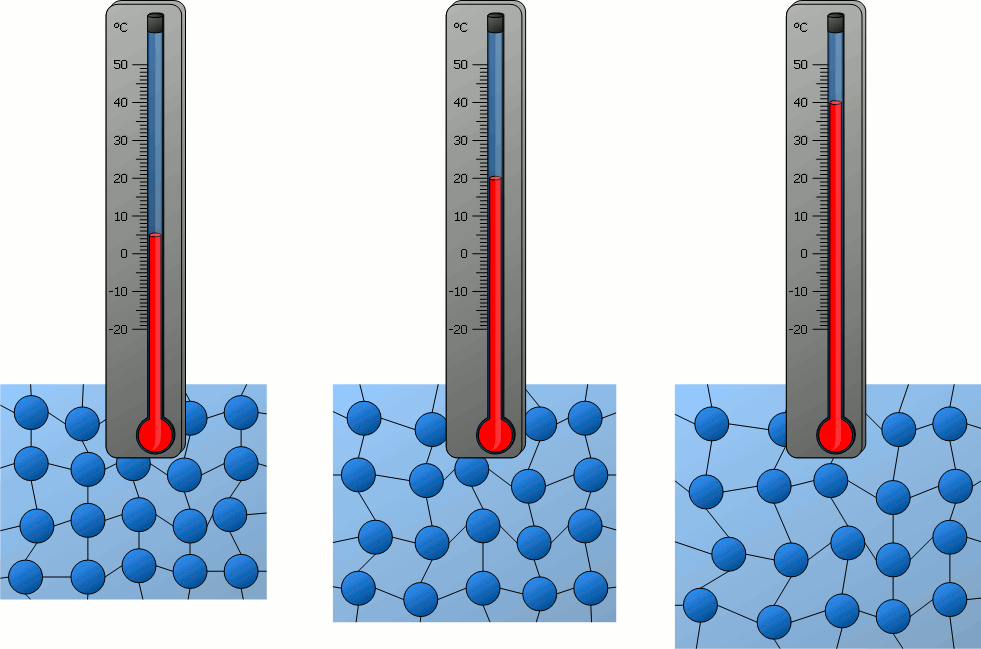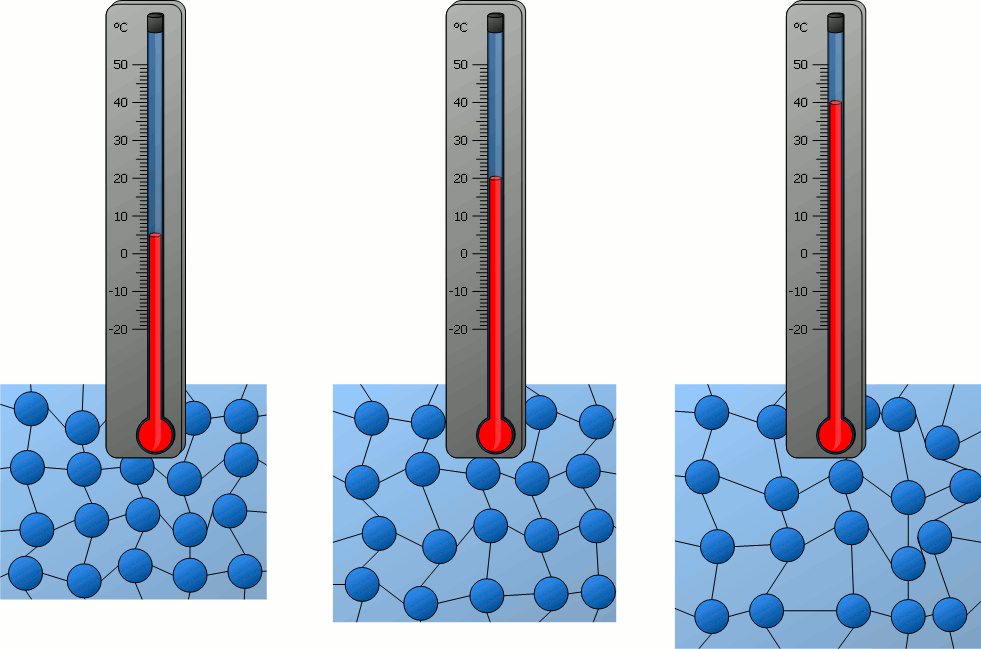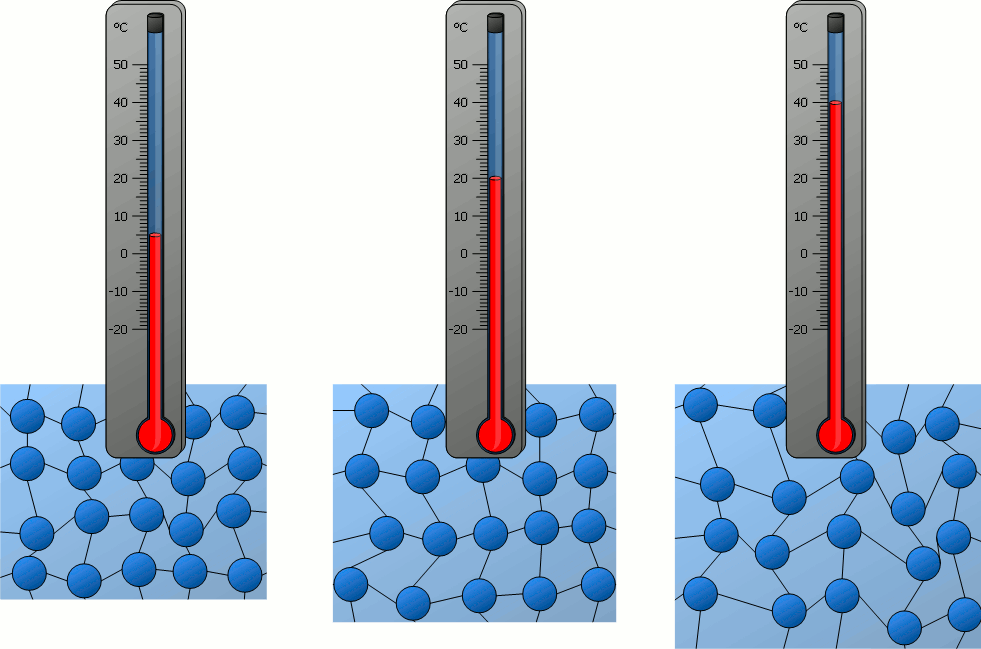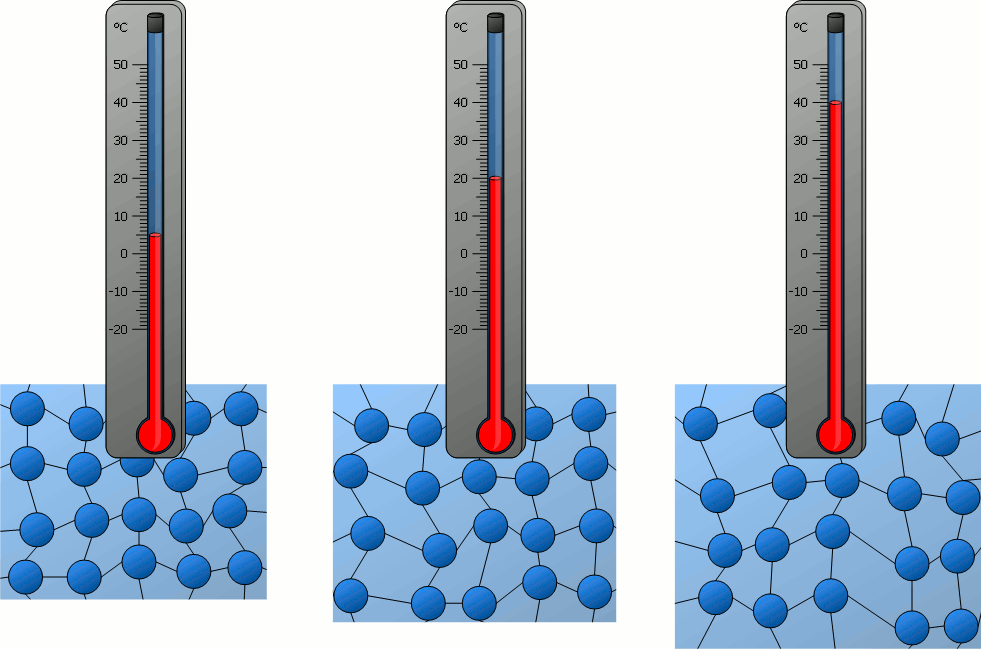
All matter is made up of particles (atoms, molecules, or ions).
These particles are constantly in motion:
- In solids: vibrate in place
- In liquids: move and slide past each other
- In gases: move quickly and randomly
As the temperature increases, particles move faster because they gain kinetic energy.
As the temperature decreases, particle motion slows down – their kinetic energy decreases.
Temperature and Kinetic Energy
Temperature is a measure of the average kinetic energy of the particles in a substance.

The kinetic energy of a particle is proportional to the square of its speed:
\( E_k \propto v^2 \)
When temperature increases:
- Particles move faster
- They collide more often and with greater energy
Absolute Zero: $-273^\circ \text{C}$
There is a lowest possible temperature, called absolute zero.
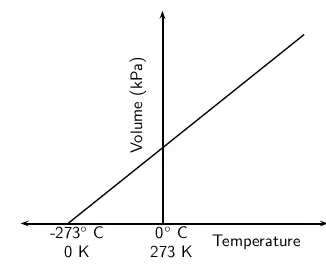
Absolute zero = \( -273^\circ \text{C} \) = \( 0\, \text{K} \)
At this temperature:
- Particles have no kinetic energy
- There is no movement – even vibrations stop
Absolute zero is the starting point of the Kelvin scale.
| Condition | Particle Behavior | Kinetic Energy |
|---|---|---|
| Increase in temperature | Particles move faster and collide more frequently | Increases |
| Decrease in temperature | Particles move more slowly and collide less often | Decreases |
| At absolute zero (0 K) | Particles stop moving completely | Minimum (zero motion) |
Example:
A container of gas is heated. Explain how this affects the particles in terms of motion and kinetic energy.
▶️ Answer/Explanation
When the temperature increases, the particles gain energy and move faster. Their average kinetic energy increases.
As a result, particles collide more frequently and with greater force, increasing the pressure (if volume is fixed).
\( \boxed{\text{Temperature ↑ ⇒ Particle speed ↑ ⇒ Kinetic energy ↑}} \)
Example:
What happens to the motion of particles as the temperature of a gas approaches absolute zero?
▶️ Answer/Explanation
As temperature decreases, the kinetic energy of the gas particles decreases. The particles move more slowly.
At absolute zero (\( -273^\circ \text{C} \) or \( 0\, \text{K} \)), the particles have zero kinetic energy and stop moving completely.
\( \boxed{\text{At 0 K, particle motion = 0}} \)
Gas Pressure and Particle Motion
Gas Pressure and Particle Motion
Gas pressure
Gas pressure is caused by the collisions of gas particles with the walls of the container.

- Each time a particle hits the surface, it exerts a tiny force.
- The total pressure is the sum of all these tiny forces over the surface area.
\( \text{Pressure} = \frac{\text{Total force}}{\text{Area}} \)
How does particle motion create pressure?

- In a gas, particles move randomly and at high speeds.
- They collide with the walls of the container frequently and with force.
- Each collision transfers momentum to the wall, creating pressure.
- The more frequent and forceful the collisions, the higher the pressure.
Factors affecting gas pressure
- Temperature: Increasing temperature gives particles more kinetic energy.
- Particles move faster
- Collisions with walls become more frequent and forceful
- \( \Rightarrow \) Pressure increases
- Volume (at constant temperature): Decreasing volume forces particles into a smaller space.
- Particles hit the walls more often
- \( \Rightarrow \) Pressure increases
- Number of particles (mass of gas): More particles means more collisions.
- More total force on walls
- \( \Rightarrow \) Pressure increases
Example:
A student uses a pump to inflate a bicycle tyre. As the air is pumped in, the volume inside the tyre remains nearly constant, but the number of gas particles increases. Explain why the pressure inside the tyre increases using the particle model.
▶️ Answer/Explanation
Adding more particles
Each time the pump pushes air in, more gas particles enter the tyre.
Collisions increase
With more particles in the same volume, the number of collisions with the tyre walls increases.
More total force on surface
These extra collisions mean more total force is exerted on the inner walls of the tyre.
Pressure increases
Since \( \text{Pressure} = \frac{\text{Force}}{\text{Area}} \), the increased force from more frequent collisions leads to increased pressure.
\( \boxed{\text{More particles in same volume ⇒ more collisions ⇒ higher pressure}} \)
Gas Pressure in Terms of Force per Unit Area
Pressure is defined as the force applied per unit area.
\( \text{Pressure} = \frac{\text{Force}}{\text{Area}} \)
- In gases, this force is created by particles colliding with the walls of the container.
- Each collision transfers momentum to the wall and exerts a tiny force.
- Millions of these collisions per second produce a measurable pressure.
How gas particles create pressure
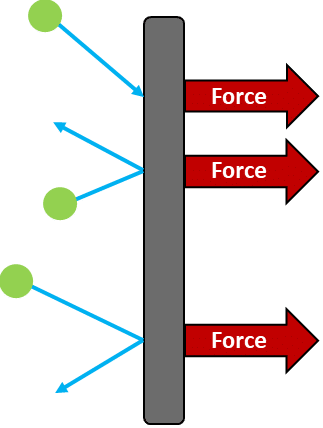
- Gas particles move rapidly in all directions due to their kinetic energy.
- When they strike the container walls, they exert a force on the surface.
- The sum of all these forces over the surface area gives the gas pressure.
\( \text{Gas pressure} = \frac{\text{Total force of collisions}}{\text{Surface area of container}} \)
What causes changes in pressure?
Increase in temperature:
| Decrease in volume (same number of particles):
|
Example:
A sealed rigid container of gas is heated. Explain in terms of particle collisions and force per unit area why the gas pressure increases.
▶️ Answer/Explanation
Particle speed increases
Heating the gas increases the kinetic energy of the particles, so they move faster.
Collisions become more frequent and forceful
Faster-moving particles collide with the walls more frequently and with greater force.
Increase in total force on walls
The total force from all these particle collisions increases.
Pressure increases
Since \( \text{Pressure} = \frac{\text{Force}}{\text{Area}} \), and the area stays constant, the increase in total force results in higher pressure.
\( \boxed{\text{Faster collisions ⇒ Greater force ⇒ Higher pressure}} \)
Example:
Gas particles exert a total force of \( 600\, \text{N} \) on the inside surface of a container. The total area of the inside surface is \( 0.75\, \text{m}^2 \). Calculate the pressure exerted by the gas on the container walls. Give your answer in pascals (Pa).
▶️ Answer/Explanation
Write the formula for pressure
\( P = \frac{F}{A} \)
Substitute the values
\( P = \frac{600}{0.75} \)
Calculate
\( P = 800\, \text{Pa} \)
Brownian Motion and the Kinetic Particle Model
Brownian Motion and the Kinetic Particle Model
Brownian motion is the random, erratic movement of tiny visible particles suspended in a fluid (gas or liquid).
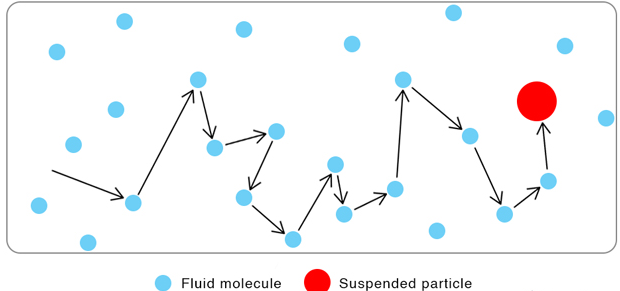
- Examples of such particles: smoke particles in air, pollen grains in water.
- This motion is observed under a microscope and appears as jittery or zigzag movement.
Cause of Brownian Motion
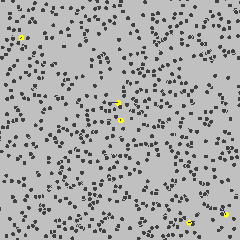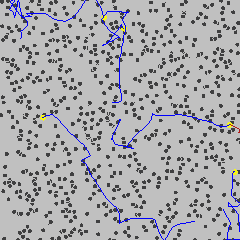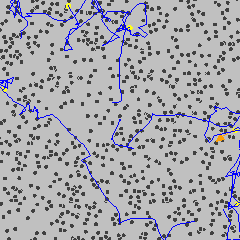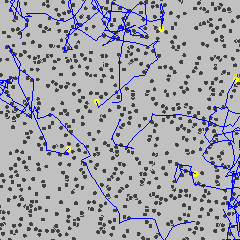
- The motion is caused by collisions with smaller, invisible particles in the fluid.
- These are the atoms or molecules of the gas or liquid, which are:
- Very small
- Light
- Moving quickly and randomly
- When these fast-moving atoms or molecules collide unevenly with a larger microscopic particle, they push it in random directions.
Distinguishing Between Terms
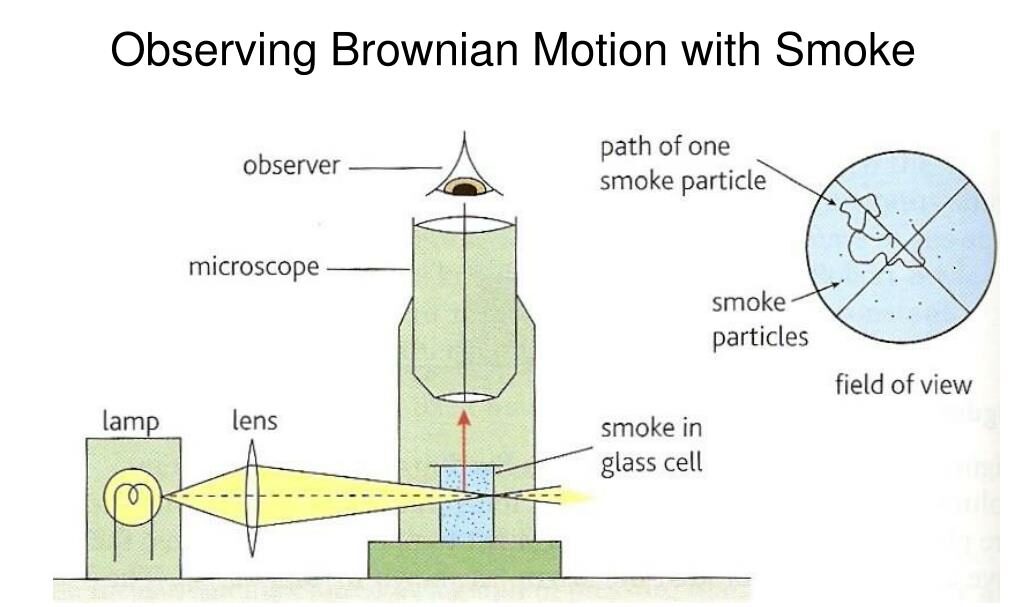
- Microscopic particles:
- Larger than atoms or molecules, but still small (e.g. smoke or pollen)
- Can be seen under a microscope
- They undergo Brownian motion
- Atoms or molecules:
- Constitute the fluid (air or water)
- Move rapidly and randomly due to their kinetic energy
- Too small to be seen with a microscope
- Cause the motion of microscopic particles through collisions
Explanation using the Kinetic Particle Model
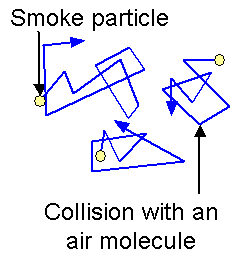
- According to the kinetic particle model:
- All matter is made of particles (atoms or molecules)
- These particles are in constant random motion
- In Brownian motion:
- The microscopic particles (e.g. pollen grains, smoke particles) are large compared to gas or liquid molecules
- These microscopic particles are bombarded unevenly by faster-moving, invisible molecules of the fluid
- Because the collisions are random and uneven:
- The microscopic particle is constantly pushed in random directions
- This results in its observed erratic motion
Importance of Brownian Motion
- It provides evidence for the existence of atoms and molecules.
- The motion shows that the invisible particles (atoms or molecules) in a gas or liquid are moving randomly and exerting force during collisions.
- Supports the idea that these particles have kinetic energy and are always in motion, even if we cannot see them directly.
Example:
When viewed under a microscope, smoke particles in still air are seen to move in a continuous, random, and jerky motion. Explain this observation using the kinetic particle model.

▶️ Answer/Explanation
The smoke particles are microscopic particles. They are large enough to be seen under a microscope but small enough to be moved by collisions.
The air around the smoke particles contains invisible, fast-moving molecules of gas (atoms or molecules).
These light, fast-moving air molecules collide randomly and unevenly with the smoke particles.
Because the collisions are unbalanced and random, the smoke particles move in a jittery, zigzag motion.
This supports the kinetic particle model, which says that particles of a gas are constantly moving and colliding.
Example:
When viewed under a microscope, pollen grains suspended in water are seen to move in a random, jerky motion. Explain this observation in terms of the kinetic particle model.

▶️ Answer/Explanation
The pollen grain is a microscopic particle – visible under a microscope but much larger than the surrounding water molecules.
The water is made of many tiny, invisible molecules that are in constant random motion.
The pollen grain is bombarded unevenly by the fast-moving water molecules. These collisions are random and unbalanced.
As a result, the pollen grain moves in a continuous, random, zigzag motion. This motion is called Brownian motion.