CIE IGCSE Physics (0625) Radiation Study Notes - New Syllabus
CIE IGCSE Physics (0625) Radiation Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of Radiation
Key Concepts:
- Thermal Radiation
- Thermal Balance and Temperature Change
- Earth’s Temperature and Radiation Balance
- Good , Bad emitters & Good , Bad absorbers Experiments
- Factors Affecting the Rate of Emission of Infrared Radiation
Thermal Radiation
1. Thermal Radiation Is Infrared Radiation s to transfer energy, thermal radiation can travel through a vacuum.
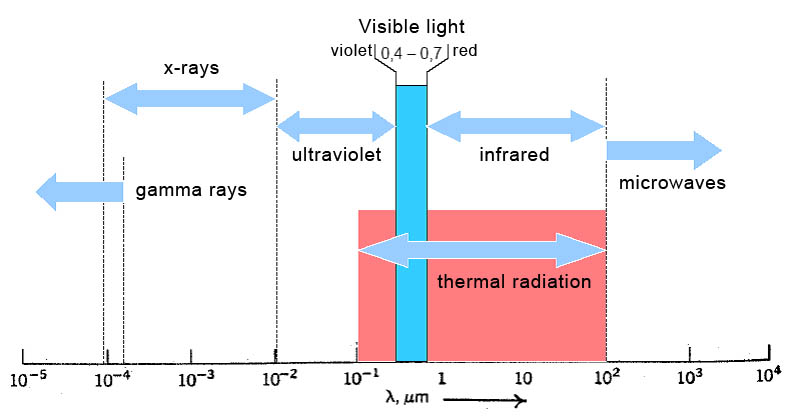 |  |
- This is because radiation is part of the electromagnetic spectrum – it behaves like light and does not need particles to move.
- This is how thermal energy from the Sun reaches Earth through space, which is nearly a perfect vacuum.
Note: Radiation is the only form of thermal energy transfer that works in space or across airless gaps.
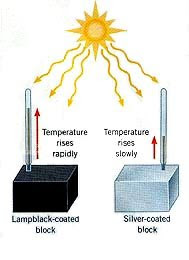
Effect of Surface Colour and Texture on Infrared Radiation
| Surface Type | Absorption of IR | Emission of IR | Reflection of IR |
|---|---|---|---|
| Black and Dull | Very Good Absorber | Very Good Emitter | Very Poor Reflector |
| White and Shiny | Poor Absorber | Poor Emitter | Good Reflector |
Explanations:
- Black, dull surfaces absorb and emit infrared radiation effectively but reflect poorly.
- White, shiny surfaces reflect most infrared radiation and are poor absorbers and emitters.
Examples:
- A black car gets hotter in sunlight than a white car.
- Radiators are often painted black to emit more heat.
- Space blankets use shiny foil to reflect body heat back to the person.
Example:
A thermal imaging camera is used to view a room. It shows warm objects like people, laptops, and lamps glowing brightly, while colder objects like walls and books appear darker. Why?
▶️ Answer/Explanation
All objects emit infrared radiation, even if they are not visibly hot.
Warm objects emit more infrared radiation than cooler ones, which the thermal camera detects.
This proves that all objects emit thermal radiation, and hotter objects emit more of it.
Conclusion: Thermal radiation is emitted by all objects depending on their temperature.
Example:
Two metal plates of the same size and material are heated to the same temperature. One plate is painted dull black and the other is shiny silver. After they are removed from the heat source and placed in a cold room, the temperature of the black plate drops faster than that of the silver plate.
▶️ Answer/Explanation
The dull black plate is a much better emitter of infrared radiation than the shiny silver plate.
Both plates started at the same temperature, but the black surface radiates energy away more quickly due to its surface texture and colour.
This is because black, matte surfaces are good emitters, while shiny, reflective surfaces are poor emitters.
Conclusion: Surface colour and texture affect how quickly objects emit (and also absorb) thermal radiation — dull black surfaces emit and absorb more, shiny white surfaces emit and absorb less.
Thermal Balance and Temperature Change
Thermal Balance and Temperature Change
1. Constant Temperature Condition:
- For an object to stay at a constant temperature, it must transfer energy away at the same rate that it receives energy.
- This applies to any method of energy transfer – conduction, convection, or radiation.

Example: A person sitting in sunlight may absorb heat from the Sun but also lose heat through radiation and convection. If the energy gained equals the energy lost, their body temperature stays constant.
2. What Happens If the Rates Are Unequal:
- If the object receives more energy than it transfers away:
- Its internal energy increases.
- Its temperature will rise.
- If the object transfers away more energy than it receives:
- Its internal energy decreases.
- Its temperature will fall.

Example: A cup of tea left on a table loses more energy (to the air) than it gains – so it cools down.
Example: A metal plate placed under a strong lamp may gain more energy from the lamp than it can radiate away, so it heats up.
Example:
On a sunny day, a metal plate is placed in direct sunlight. Its temperature increases for several minutes and then remains steady at 60°C, even though sunlight continues to shine on it. Give the reason why the temperature stops increasing.
▶️ Answer/Explanation
Initially, the plate absorbs more energy from sunlight than it loses, so its temperature rises.
Eventually, it emits infrared radiation and transfers energy to the air (by convection) at the same rate it absorbs energy from the Sun.
This is when the energy input equals the energy output – a state called thermal equilibrium.
Conclusion: The temperature remains constant when the rate of energy gain equals the rate of energy loss.
Earth's Temperature and Radiation Balance
Earth’s Temperature and Radiation Balance
The Earth’s temperature is controlled by a balance between:
- Incoming radiation from the Sun (mostly visible light and short-wave infrared)
- Outgoing radiation emitted by the Earth (mainly long-wave infrared)

1. When the Incoming and Outgoing Radiation Are Equal:
- The Earth is in thermal balance.
- The global average temperature remains stable.
Example: Over long periods, if the energy absorbed from the Sun equals the energy emitted, Earth’s temperature stays constant.
2. When Incoming Radiation Is Greater Than Outgoing Radiation:
- The Earth absorbs more energy than it emits.
- This causes the average global temperature to increase (global warming).
Causes:
- Increased greenhouse gases (e.g., CO2, methane) trap outgoing radiation.
- More energy is retained in the atmosphere.
3. When Outgoing Radiation Is Greater Than Incoming Radiation:
- The Earth loses more energy than it gains.
- This causes the average temperature to decrease (cooling).
Possible Causes:
- Volcanic ash or dust reflecting sunlight back into space.
- Reduction in solar output.
Conclusion: The Earth’s climate and average temperature depend on maintaining a delicate balance between incoming solar radiation and the radiation emitted back into space. Disruption to this balance causes either global warming or global cooling.
Example:
The Earth absorbs energy from the Sun as visible and infrared radiation. At the same time, it emits infrared radiation back into space. In recent decades, the Earth’s average temperature has been rising. Explain why the temperature is increasing.
▶️ Answer/Explanation
Normally, the Earth maintains a stable average temperature when the energy it absorbs from the Sun is equal to the energy it radiates back into space.
However, greenhouse gases (like carbon dioxide and methane) trap more of the outgoing infrared radiation in the atmosphere.
This reduces the rate of energy loss, while energy input from the Sun remains the same.
As a result, the Earth absorbs more energy than it emits, leading to an overall rise in temperature — this is known as global warming.
Conclusion: If the Earth receives more energy than it emits, its temperature increases. If it emits more than it receives, its temperature decreases.
Good , Bad emitters & Good , Bad absorbers Experiments
Experiment 1: Distinguishing Between Good and Bad Emitters of Infrared Radiation
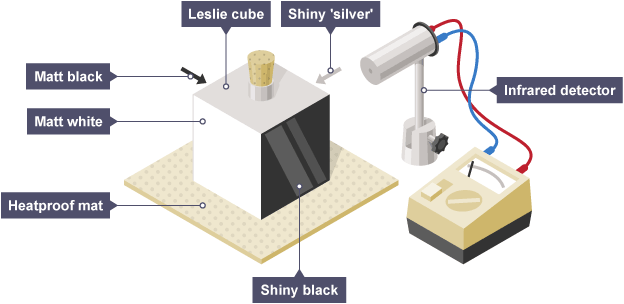
Apparatus:
- Leslie cube (a metal cube with four different surfaces: black, white, shiny, and dull)
- Hot water
- Infrared (IR) detector or thermopile
Method:
- Fill the Leslie cube with hot water so all surfaces are at the same temperature.
- Use the infrared detector to measure the infrared radiation emitted from each face of the cube from the same distance.
Observations:
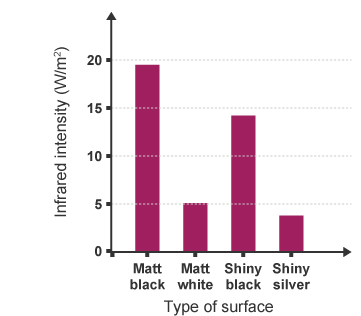
- The black, dull surface emits the most infrared radiation.
- The white or shiny surface emits the least infrared radiation.
Results
Record results in a suitable table. The table below shows some example results:
| Surface type | Infrared intensity (W/m²) |
|---|---|
| matt black | 19.5 |
| matt white | 5.1 |
| shiny black | 14.2 |
| shiny silver | 3.8 |
Analysis
- Plot a bar chart to show the results. Make sure each bar is the same width and labelled clearly to show which surface it represents.

Conclusion:
- Black, dull surfaces are the best emitters of infrared radiation.
- White or shiny surfaces are poor emitters of infrared radiation.
Experiment 2: Distinguishing Between Good and Bad Absorbers of Infrared Radiation
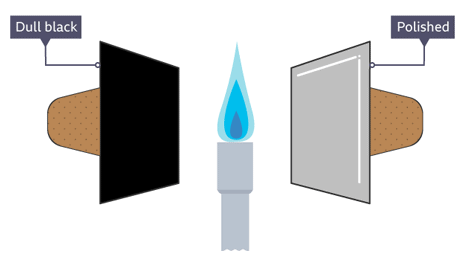
Apparatus:
- Two identical metal cans or plates: one painted black and dull, the other white or shiny
- Infrared lamp (or a strong lamp like a spotlight)
- Thermometers (placed inside or attached to each can)
Method:
- Place the cans equal distances from the infrared lamp.
- Turn on the lamp and record the temperature of each can every minute for 10 minutes.
Observations:
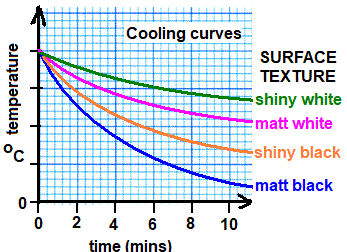
- The temperature of the black can rises more quickly than the white can.
Conclusion:
- Black, dull surfaces absorb infrared radiation better than shiny or white ones.

Surface Texture Cooling Rate Emitter Quality Matt Black Fastest Very good Shiny Black Second fastest Good Matt White Second slowest Poor Shiny White Slowest Very poor
Example:
A Leslie cube is filled with boiling water. Each of its four vertical faces has a different surface: black matte, white matte, shiny metal, and dull grey. An infrared detector is used to measure the radiation emitted by each face. Which surface will show the highest radiation reading?
A. Shiny metal
B. White matte
C. Dull grey
D. Black matte
▶️ Answer/Explanation
The black matte surface is the best emitter of infrared radiation because it is both dark and dull. Shiny and white surfaces reflect more and emit less.
Correct Answer: D
Example:
Two identical metal cans are placed an equal distance from a strong infrared heat lamp. One can is painted black and dull, the other is shiny silver. Thermometers inside each can record the temperature over time. Which result is expected?
A. Both cans heat up at the same rate
B. The shiny can heats up faster
C. The black can heats up faster
D. The shiny can becomes hotter but more slowly
▶️ Answer/Explanation
The black, dull surface absorbs more infrared radiation than the shiny silver surface. Therefore, it heats up faster under the same energy input.
Correct Answer: C
Factors Affecting the Rate of Emission of Infrared Radiation
Factors Affecting the Rate of Emission of Infrared Radiation
1. Surface Temperature:
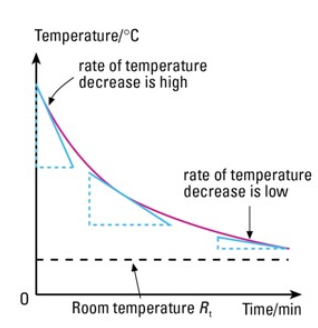
- The higher the surface temperature of an object, the more infrared radiation it emits per second.
- As the temperature increases, the particles in the object vibrate or move faster, so they lose more energy as radiation.
- Hotter objects emit more energy and at shorter wavelengths.
Example: A hot iron emits more infrared radiation than a warm one; a glowing filament emits both IR and visible light due to its very high temperature.
2. Surface Area:
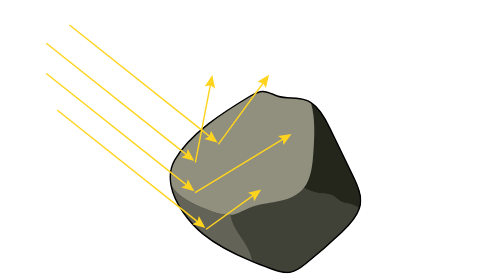
- The larger the surface area of an object, the more space it has to emit radiation from.
- Larger objects radiate more energy per second than smaller objects at the same temperature.
- This is because more particles are exposed to the surroundings, allowing more emission points.
Example: A large metal plate cools down faster than a small one at the same temperature, because it radiates more energy due to its larger surface area.
Conclusion:
- The rate of infrared radiation emission increases with both surface temperature and surface area.
- This principle is important in designing radiators, cooling systems, and spacecraft surfaces.
Example:
Two metal objects are placed in a cold room. Object A is small and heated to 80°C, while Object B is large but only heated to 50°C. After 10 minutes, it is observed that Object A cools down more slowly than Object B. Explain why Object B loses energy faster, even though it is cooler.
▶️ Answer/Explanation
The rate of emission of infrared radiation depends on two main factors: surface temperature and surface area.
Although Object A is hotter, it has a smaller surface area, so it emits less total radiation.
Object B, despite being cooler, has a much larger surface area, so it emits more energy per second overall.
Conclusion: A larger surface area can cause a cooler object to emit radiation faster than a hotter object with a small area.