CIE IGCSE Physics (0625) Radioactive decay Study Notes - New Syllabus
CIE IGCSE Physics (0625) Radioactive decay Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of Radioactive decay
Key Concepts:
- Radioactive decay
- Change of Element During Radioactive Decay
Radioactive Decay
Radioactive Decay
Radioactive decay is a spontaneous and random nuclear process in which an unstable atomic nucleus loses energy by emitting radiation. This helps the nucleus reach a more stable configuration.

Characteristics of Radioactive Decay:
- Spontaneous: It happens on its own – not triggered by external factors such as temperature, pressure, or chemical reactions.
- Random: We cannot predict when a specific nucleus will decay – only the probability of decay over time.
Why Do Some Nuclei Become Unstable?
Nuclei become unstable due to one or both of the following reasons:
- Too many neutrons: An imbalance in the neutron-to-proton ratio causes instability. This is common in beta decay.
- Too many nucleons (protons + neutrons): Very heavy nuclei (like uranium or radon) are unstable due to their large mass and may emit alpha particles.
Connection with Isotopes:
- Isotopes are atoms of the same element with different numbers of neutrons.
- Some isotopes are radioactive isotopes (radioisotopes).
- A radioisotope becomes unstable when it has:
- Too many neutrons (e.g., carbon-14)
- Too many protons (rare, but possible)
- A nucleus that is simply too massive (e.g., uranium-238)
Types of Radiation Emitted During Decay:

Alpha (α) decay:
- Emission of a helium nucleus (2 protons + 2 neutrons)
- Mass number decreases by 4, atomic number by 2
- Common in heavy nuclei like uranium, radium
Beta (β⁻) decay:
- A neutron changes into a proton and emits a high-speed electron (β⁻)
- Atomic number increases by 1, mass number unchanged
- Common in isotopes with too many neutrons (e.g., carbon-14)
Gamma (γ) radiation:
- High-energy electromagnetic wave emitted by a nucleus after α or β decay
- No change to mass number or atomic number
- Reduces the energy of the nucleus (de-excitation)
Change of Element During Radioactive Decay
Change of Element During Radioactive Decay
During both alpha (α) decay and beta (β⁻) decay, the structure of the nucleus changes in such a way that the atomic number (number of protons) changes.
- Since the atomic number defines the element, a change in atomic number means the atom becomes a different element.
Case 1: Alpha Decay

- The nucleus emits an alpha particle \( (^4_2\text{He}) \)
- The atomic number decreases by 2 → the element changes

Example: Uranium-238 (\( ^{238}_{92}U \)) becomes Thorium-234 (\( ^{234}_{90}Th \))
Case 2: Beta Decay (β⁻)
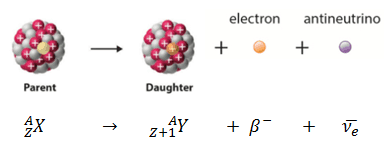
- A neutron changes into a proton and an electron is emitted
- The atomic number increases by 1 → the element changes
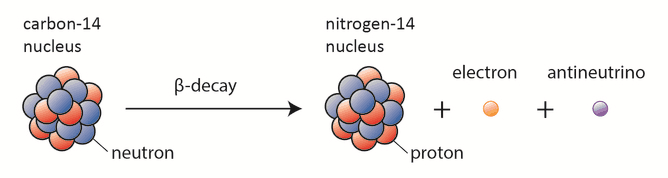
Example: Carbon-14 (\( ^{14}_6C \)) becomes Nitrogen-14 (\( ^{14}_7N \))
Therefore, during both α-decay and β⁻-decay, the nucleus transforms into a different element.
| Decay Type | Change in Nucleus | Change in Atomic No. | Change in Mass No. | Purpose / Effect |
|---|---|---|---|---|
| Alpha (α) | Loss of 2 protons & 2 neutrons | –2 | –4 | Reduces large nuclear mass |
| Beta (β⁻) | Neutron → proton + electron | +1 | 0 | Corrects neutron excess |
| Gamma (γ) | Nucleus loses excess energy | 0 | 0 | Releases energy after α or β decay |
Example :
A nucleus of Plutonium-239 undergoes alpha decay. Identify the new element formed and write the nuclide equation.
▶️ Answer/Explanation
Step 1: Write the original nuclide
\( \mathrm{^{239}_{94}Pu} \)
Step 2: Subtract the alpha particle \( \mathrm{^4_2He} \)
New mass number: \( 239 – 4 = 235 \)
New atomic number: \( 94 – 2 = 92 \)
Step 3: Identify the new element
Atomic number 92 = Uranium (U)
\( \boxed{\mathrm{^{239}_{94}Pu \rightarrow\ ^{235}_{92}U\ +\ ^4_2He}} \)
The element changes from Plutonium (Pu) to Uranium (U).
Example :
A nucleus of Phosphorus-32 undergoes beta decay. Identify the new element formed and write the nuclide equation.
▶️ Answer/Explanation
Step 1: Write the original nuclide
\( \mathrm{^{32}_{15}P} \)
Step 2: In beta decay, a neutron becomes a proton and emits an electron \( \mathrm{^0_{-1}e} \)
New mass number: \( 32 \) (no change)
New atomic number: \( 15 + 1 = 16 \)
Step 3: Identify the new element
Atomic number 16 = Sulfur (S)
\( \boxed{\mathrm{^{32}_{15}P \rightarrow\ ^{32}_{16}S\ +\ ^0_{-1}e}} \)
The element changes from Phosphorus (P) to Sulfur (S).
Example:
Write the decay equation for the alpha decay of Uranium-238.
▶️ Nuclide Equation & Explanation
Parent Nucleus: \( \mathrm{^{238}_{92}U} \)
Alpha particle: \( \mathrm{^4_2He} \)
Daughter Nucleus: \( \mathrm{^{234}_{90}Th} \)
\( \boxed{\mathrm{^{238}_{92}U \rightarrow\ ^{234}_{90}Th\ +\ ^4_2He}} \)
Example :
Write the decay equation for the beta decay of Carbon-14.
▶️ Nuclide Equation & Explanation
Parent Nucleus: \( \mathrm{^{14}_6C} \)
Beta particle: \( \mathrm{^0_{-1}e} \)
Daughter Nucleus: \( \mathrm{^{14}_7N} \)
\( \boxed{\mathrm{^{14}_6C \rightarrow\ ^{14}_7N\ +\ ^0_{-1}e}} \)
Example :
After beta decay, Barium-137m (a metastable nucleus) emits gamma radiation. Write the equation for this.
▶️ Nuclide Equation & Explanation
Excited Nucleus: \( \mathrm{^{137m}_{56}Ba} \)
Gamma ray: \( \gamma \)
Stable Nucleus: \( \mathrm{^{137}_{56}Ba} \)
\( \boxed{\mathrm{^{137m}_{56}Ba \rightarrow\ ^{137}_{56}Ba\ +\ \gamma}} \)
Note: There is no change in atomic or mass number; only excess energy is lost.
