CIE IGCSE Physics (0625) Specific heat capacity Study Notes - New Syllabus
CIE IGCSE Physics (0625) Specific heat capacity Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of Specific heat capacity
Key Concepts:
- Temperature, Internal Energy, and Particle Kinetic Energy
- Specific Heat Capacity (c)
- Experiments to Measure Specific Heat Capacity
Temperature, Internal Energy, and Particle Kinetic Energy
Internal Energy
All matter is made of particles (atoms or molecules) that are in constant motion.Every object contains internal energy, which is the total energy of all its particles.

- It includes both:
- Kinetic Energy (KE): energy due to motion of particles (vibration, translation)
- Potential Energy (PE): energy due to forces between particles (bonds, separation)
- Internal energy = total KE + total PE of all particles in the object
Changes in Internal Energy
- Heating an object increases its internal energy.
- If the object does not change state, this energy goes into increasing the kinetic energy of particles.
- If the object is changing state (melting, boiling, condensing, etc.), the temperature stays constant but:
- The energy increases the potential energy (breaking or forming bonds).

Link Between Temperature and Internal Energy
Temperature is directly related to the average kinetic energy of the particles.
- As temperature increases:
- Particles move faster (more KE)
- Internal energy increases due to higher KE
- This happens in all states of matter:

- Solids: particles vibrate more vigorously
- Liquids: particles move more freely and spread slightly
- Gases: particles move rapidly and randomly
Example:
A metal block is heated from 20°C to 60°C. Explain what happens to its internal energy and the motion of its particles.
▶️ Answer/Explanation
Heating increases the temperature of the metal block.
As temperature rises, the particles inside the solid gain kinetic energy.
The particles vibrate more rapidly about their fixed positions.
Since kinetic energy increases, the internal energy of the metal block also increases.
Heating the block increases the average kinetic energy and internal energy of its particles.
Specific Heat Capacity (c)
Specific Heat Capacity (c)
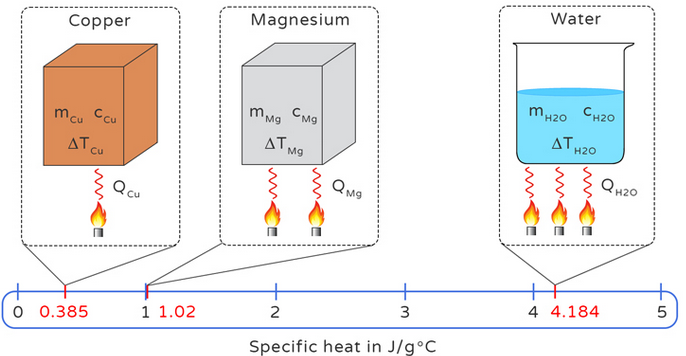
The specific heat capacity of a substance is the amount of energy required to raise the temperature of 1 kg of the substance by 1°C (or 1 K). It measures how much energy a material can store per degree of temperature increase.
Equation:
\( Q = mc\Delta T \)
- \( Q \) = energy supplied in joules (J)
- \( m \) = mass of the substance in kilograms (kg)
- \( c \) = specific heat capacity in J/kg·°C
- \( \Delta T \) = temperature change in °C or K

Important Notes:
- Specific heat capacity is different for each material.
- Water has a high specific heat capacity: it takes a lot of energy to heat up.
- Metals like copper or aluminum heat up quickly (lower \( c \)) because they require less energy per °C.
- Applications:
- Heating systems and radiators (use metals)
- Water in hot-water bottles (stores heat well)
- Cooling engines using water (absorbs heat effectively)
Example:
How much energy is needed to raise the temperature of 2.0 kg of water from 20°C to 60°C? (Specific heat capacity of water = 4200 J/kg·°C)
▶️ Answer/Explanation
Use the equation: \( Q = mc\Delta T \)
Identify the values:
\( m = 2.0\, \text{kg} \)
\( c = 4200\, \text{J/kg·°C} \)
\( \Delta T = 60 – 20 = 40\, \text{°C} \)
Substitute into the equation:
\( Q = 2.0 \times 4200 \times 40 = 336000\, \text{J} \)
| Substance | Specific Heat Capacity \( (c) \) | State at Room Temp | Comments |
|---|---|---|---|
| Water | \( 4200\, \text{J/kg·°C} \) | Liquid | Very high; stores heat well |
| Aluminium | \( 900\, \text{J/kg·°C} \) | Solid | Used in cooking utensils |
| Iron | \( 450\, \text{J/kg·°C} \) | Solid | Heats quickly; cools quickly |
| Copper | \( 385\, \text{J/kg·°C} \) | Solid | Good conductor; low \( c \) |
| Air | \( 1000\, \text{J/kg·°C} \) | Gas | Insulator; low density |
Example:
Equal masses of copper and water (1.0 kg each) are heated by 20°C. Calculate and compare the energy required to heat both. Use:
- Specific heat capacity of copper: \( c = 385\, \text{J/kg·°C} \)
- Specific heat capacity of water: \( c = 4200\, \text{J/kg·°C} \)
▶️ Answer/Explanation
Use the equation \( Q = mc\Delta T \)
For copper:
\( Q_{\text{copper}} = 1.0 \times 385 \times 20 = 7700\, \text{J} \)
For water:
\( Q_{\text{water}} = 1.0 \times 4200 \times 20 = 84000\, \text{J} \)
Compare the results:
Water requires much more energy to heat up by the same temperature.
Conclusion:
\(\boxed{Q_{\text{water}} = 84000\, \text{J},\quad Q_{\text{copper}} = 7700\, \text{J}}\)
Water needs more than 10× the energy of copper for the same mass and temperature rise
Experiments to Measure Specific Heat Capacity
Method 1: Measuring Specific Heat Capacity of a Solid (e.g., a metal block)
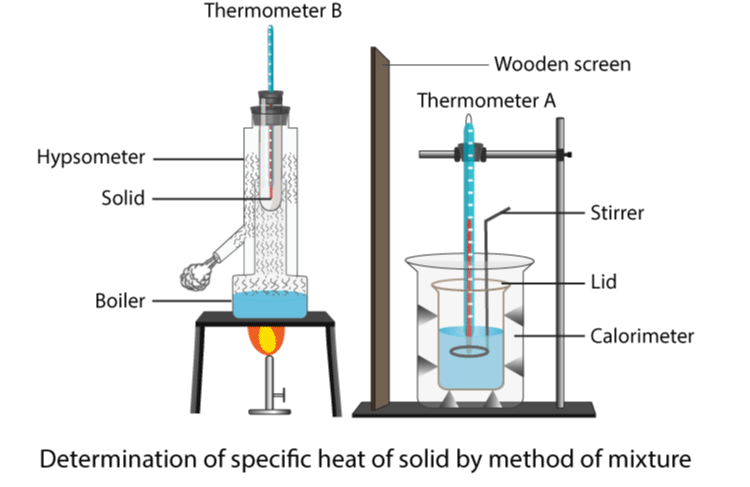
- Apparatus:
- Metal block (e.g., aluminum or copper, mass known)
- Electric immersion heater (rated in W)
- Insulating material (e.g., lagging or foam)
- Thermometer
- Stopwatch
- Ammeter and voltmeter (optional, if power not directly known)
- Procedure:
- Insert the heater and thermometer into the block (holes are pre-drilled).
- Wrap the block in insulation to minimize heat loss.
- Record the initial temperature.
- Switch on the heater and record the time it is on using a stopwatch.
- Record the final temperature after heating.
- Calculations:
- Calculate energy supplied: \( Q = P \times t \)
- Measure \( \Delta T \), and use known mass \( m \) of the block.
- Use the formula:
\( c = \dfrac{Q}{m \Delta T} \)
Method 2: Measuring Specific Heat Capacity of a Liquid (e.g., water or oil)

- Apparatus:
- Beaker with measured mass of liquid
- Electric immersion heater
- Thermometer
- Stopwatch
- Insulating container (e.g., polystyrene cup or lagged beaker)
- Lid with holes for heater and thermometer
- Procedure:
- Place the liquid and heater inside the insulated container.
- Cover with a lid to reduce energy loss by evaporation or convection.
- Record the starting temperature.
- Switch on the heater and time it accurately.
- Record the temperature rise.
- Calculations:
- Energy input: \( Q = P \times t \)
- Measure \( \Delta T \), and use known mass \( m \) of liquid.
- Use the same formula:
\( c = \dfrac{Q}{m \Delta T} \)


