CIE IGCSE Physics (0625) The three types of nuclear emission Study Notes - New Syllabus
CIE IGCSE Physics (0625) The three types of nuclear emission Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of The three types of nuclear emission
Key Concepts:
- Emission of Radiation – Spontaneous and Random Nature
- Types of Ionising Radiation: Alpha, Beta, Gamma
- Deflection of α, β, and γ Radiation in Electric and Magnetic Fields
- Ionising Effects of α, β, and γ Radiation
Emission of Radiation – Spontaneous and Random Nature
Emission of Radiation – Spontaneous and Random Nature
- When unstable atomic nuclei emit radiation (alpha, beta, or gamma), the process is said to be spontaneous and random.
“Spontaneous”
- The nucleus emits radiation without any external trigger – it happens on its own.
- You cannot force or control when a particular nucleus will decay.
“Random”
- It is impossible to predict:
- Which nucleus will decay next
- Exactly when it will decay
- In which direction the particle will be emitted
- However, for a large number of atoms, we can predict the average decay rate (using half-life).
Notes:
- Each radioactive atom behaves independently – like flipping a coin.
Example:
A radioactive sample contains millions of identical nuclei. After one minute, some atoms have decayed and others have not.
(a) Why is it impossible to predict which specific atom will decay next?
(b) Why is radiation emitted in all directions?
▶️ Answer/Explanation
(a)
Decay is a random process. Each atom has a constant probability of decaying, but there’s no way to predict which will decay next – it’s like a random event.
(b)
Radiation is emitted randomly in all directions because there is no preferred orientation inside the nucleus. The emission has no fixed pattern – it is truly random.
Types of Ionising Radiation: Alpha, Beta, Gamma
Types of Ionising Radiation: Alpha, Beta, Gamma
 Alpha Radiation (α):
Alpha Radiation (α):
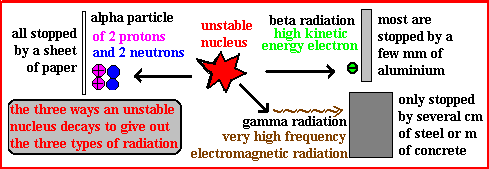
Alpha emission is the release of an alpha particle from the nucleus of an unstable atom. An alpha particle consists of 2 protons and 2 neutrons – it is essentially a helium nucleus. It is heavily charged and massive, causing it to ionise atoms strongly, but it can be stopped easily by a sheet of paper or skin.
Beta Radiation (β⁻):
Beta emission occurs when a neutron in the nucleus changes into a proton and an electron. The electron is ejected at high speed as a beta particle. Beta particles are light and fast-moving electrons that can pass through paper but are stopped by thin aluminium. Their ionising power is moderate.
Gamma Radiation (γ):
Gamma emission is the release of a high-energy electromagnetic wave (photon) from the nucleus, often after an alpha or beta decay. Gamma rays are massless and chargeless, highly penetrating but weak at ionising. They can pass through the body and most materials, requiring thick lead or concrete to be stopped.
(a) Nature of the Radiations:
| Radiation Type | Nature |
|---|---|
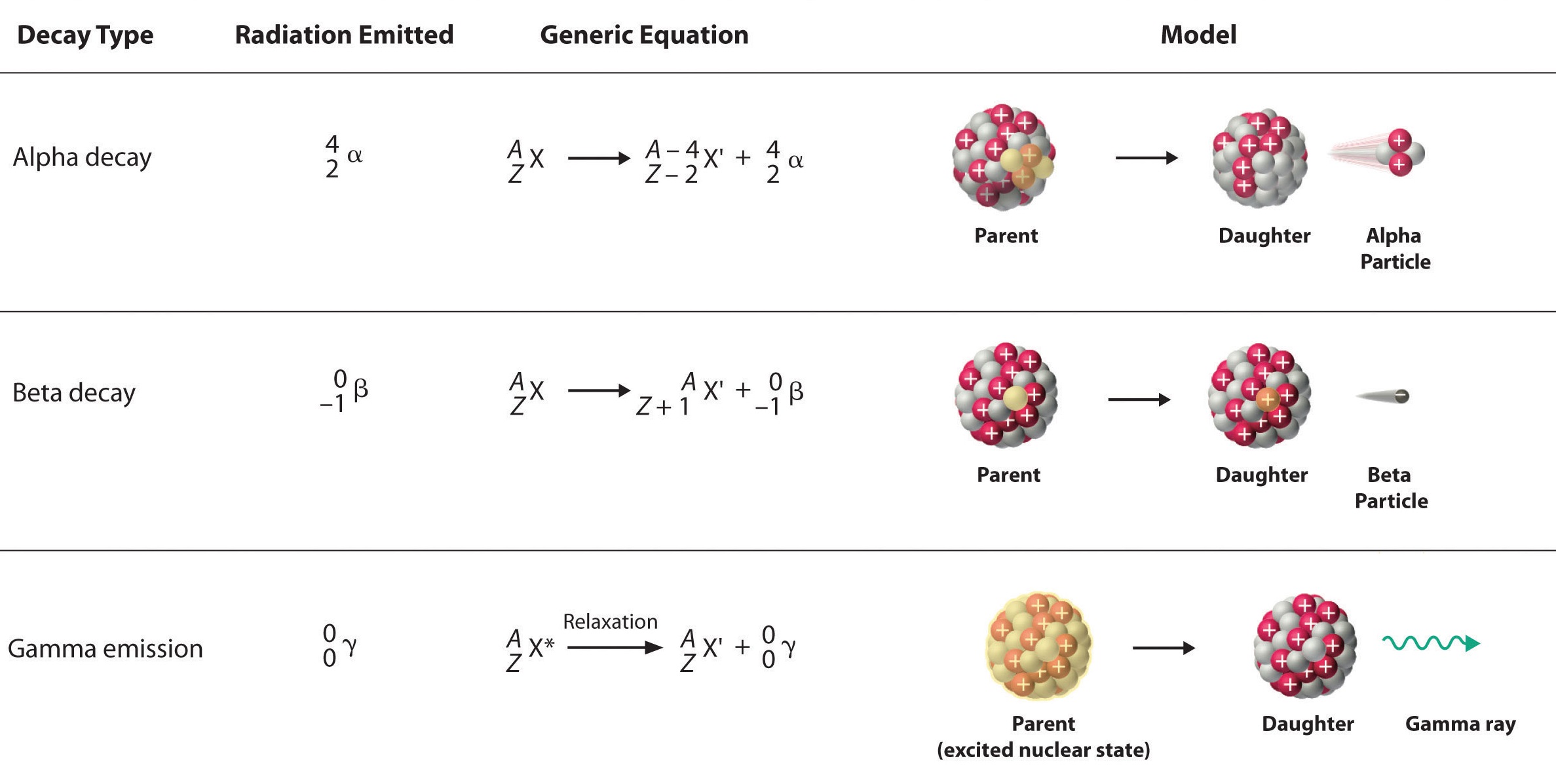
| Alpha (α) | Helium nucleus: 2 protons + 2 neutrons Symbol: \( ^4_2\text{He} \) |
| Beta (β⁻) | A high-energy electron emitted from the nucleus when a neutron turns into a proton |
| Gamma (γ) | Electromagnetic wave (very high-frequency photon) emitted after alpha or beta decay |
(b) Relative Ionising Effect:
| Radiation | Ionising Power |
|---|---|
| Alpha (α) | Very strong |
| Beta (β⁻) | Moderate |
| Gamma (γ) | Weak |
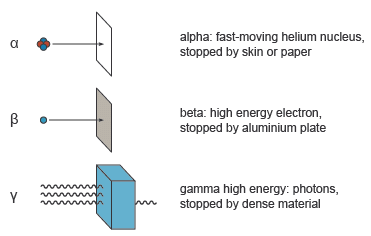
(c) Relative Penetrating Ability:
| Radiation | Penetrating Ability | Stopped By |
|---|---|---|
| Alpha (α) | Very low | A sheet of paper or skin |
| Beta (β⁻) | Moderate | Thin aluminium sheet (~5 mm) |
| Gamma (γ) | Very high | Several cm of lead or thick concrete |
Example:
A radioactive substance is emitting three types of radiation. A student uses different materials to test which types are present:
- Paper blocks one type
- Aluminium blocks another
- Only lead stops the third
(a) Identify the three types of radiation.
(b) Arrange them in order of decreasing ionising power.
▶️ Answer/Explanation
(a) Identification:
- Paper blocks alpha (α)
- Aluminium blocks beta (β⁻)
- Lead blocks gamma (γ)
(b) Ionising Power (Decreasing):
\( \boxed{\text{Alpha} > \text{Beta} > \text{Gamma}} \)
Deflection of α, β, and γ Radiation in Electric and Magnetic Fields
Deflection of α, β, and γ Radiation in Electric and Magnetic Fields
Charged particles experience a force when passing through electric or magnetic fields. The direction and amount of deflection depends on the particle’s charge and mass.
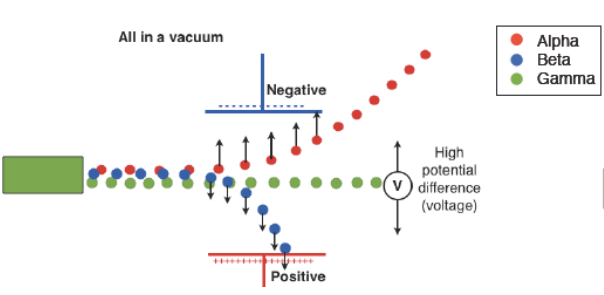
| Radiation Type | Charge | Deflection in Fields | Explanation |
|---|---|---|---|
| Alpha (α) | +2 | Deflected slightly | Heavy and positively charged – slow and less deflected |
| Beta (β⁻) | –1 | Deflected strongly | Very light and negatively charged – deflected in the opposite direction to α |
| Gamma (γ) | 0 (neutral) | Not deflected | Electromagnetic wave – no charge or mass |
Example:
A narrow beam of mixed radiation (α, β, and γ) is passed through a strong electric field between two oppositely charged plates.
(a) Describe what happens to each type of radiation.
(b) How would you use the resulting pattern to identify each radiation?
▶️ Answer/Explanation
(a)
- Alpha (α): Deflected slightly toward the negative plate
- Beta (β⁻): Deflected strongly toward the positive plate
- Gamma (γ): Continues straight, unaffected
(b)
- The most deflected trace is β-particles (lightest and negatively charged)
- The slightly deflected trace is α-particles (heavier, positively charged)
- The undeviated path is γ-rays (no charge)
Ionising Effects of α, β, and γ Radiation
Ionising Effects of α, β, and γ Radiation
Ionisation is the process of knocking electrons out of atoms to form ions. The ability of radiation to ionise depends on how much energy it transfers and how strongly it interacts with matter.
Ionising Power (From Highest to Lowest):
- Alpha (α) – Very Strong Ioniser
- Beta (β⁻) – Moderate Ioniser
- Gamma (γ) – Weak Ioniser
Ionising Effects of Radiation Based on Kinetic Energy and Electric Charge
| Radiation Type | Role of Kinetic Energy | Role of Electric Charge |
|---|---|---|
| Alpha (α) | High mass, moves slowly Carries a lot of kinetic energy Transfers large energy to atoms → strong ionisation | Charge of +2 Strong attraction to electrons High charge = strong ionising power |
| Beta (β⁻) | Low mass, fast-moving Moderate kinetic energy Weaker ionisation than alpha due to lower energy transfer | Charge of –1 Interacts with electrons but not as strongly Lower ionisation due to lower charge and mass |
| Gamma (γ) | No mass (wave) Transfers energy indirectly (photon interaction) Very weak ionisation | No charge Does not interact electrically with electrons Very poor ionising ability |
Example:
Three types of radiation—α, β, and γ—are emitted by a radioactive source. A detector measures the number of ionised air particles in a sealed chamber for each type. The results show that alpha causes the most ionisation, followed by beta, and gamma causes very little.
Using your knowledge of particle physics, explain why alpha radiation causes more ionisation than beta and gamma radiation, referring to:
(a) the particle’s kinetic energy
(b) the particle’s electric charge
▶️ Answer/Explanation
(a) Alpha particles are massive and carry a relatively large amount of kinetic energy despite being slower than beta particles. Because of their mass, they lose energy quickly as they collide with atoms and transfer energy efficiently, causing more ionisation per unit distance.
(b) Alpha particles have a charge of +2, which strongly attracts electrons from surrounding atoms. This double positive charge makes them much more likely to ionise atoms compared to beta particles (charge –1) or gamma rays (no charge).
\(\boxed{\text{High mass + high charge = greater ionising effect}}\)
for alpha radiation. Gamma rays have no charge and no mass, so they interact weakly and cause very little ionisation.
