CIE IGCSE Physics (0625) States of matter Study Notes - New Syllabus
CIE IGCSE Physics (0625) States of matter Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of States of matter
Key Concepts:
- Distinguishing Properties of Solids, Liquids, and Gases
- Changes of State
Distinguishing Properties of Solids, Liquids, and Gases
Distinguishing Properties of Solids, Liquids, and Gases
Substances can exist in three main physical states: solid, liquid, and gas. These states differ in how particles are arranged, how much energy they possess, and how they interact with each other. These differences give rise to distinct physical properties at the macroscopic level.
Solids
- Solids have a definite shape and volume.
- Particles are arranged in a tightly packed, regular pattern and can only vibrate in place.
- They are incompressible and have high density.
Liquids
- Liquids have a definite volume but take the shape of the container they are in.
- Particles are close together but arranged irregularly and can move around each other.
- They are only slightly compressible and usually less dense than solids.
Gases
- Gases have neither fixed shape nor fixed volume. They expand to fill any container.
- Particles are far apart, move freely and rapidly, and have high kinetic energy.
- They are compressible and have low density compared to solids and liquids.
Comparison of Properties
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape | Fixed | Takes shape of container | Fills entire container |
| Volume | Fixed | Fixed | Variable |
| Compressibility | Negligible | Slight | High (easily compressible) |
| Particle Arrangement | Tightly packed, regular | Close, irregular | Widely spaced, random |
| Particle Movement | Vibrate in fixed positions | Move past each other | Move freely in all directions |
| Density | High | Moderate to high | Low |
| Diffusion | Very slow | Slow | Fast |
Example:
A sealed container is filled with a gas. The gas is then slowly cooled until it becomes a liquid and then a solid.
Describe the changes that occur in the arrangement, movement, and energy of the particles during this process.
Explain how these changes affect:
- The shape and volume of the substance
- Its ability to be compressed
- The forces between the particles
▶️ Answer/Explanation
From Gas to Liquid:
- Particles lose kinetic energy and move closer together.
- The arrangement becomes more ordered but still irregular.
- Movement changes from free motion to sliding past one another.
- The gas becomes a liquid — volume becomes fixed, shape depends on container.
- Compression becomes slightly harder than for a gas due to reduced space between particles.
- Intermolecular forces increase.
From Liquid to Solid:
- Particles lose more energy and arrange in a fixed, regular structure.
- Movement becomes limited to vibrations in fixed positions.
- Volume and shape are both fixed.
- Substance becomes incompressible.
- Intermolecular forces become strongest among the three states.
Changes of State
Changes of State
Substances exist in three physical states – solid, liquid, and gas – depending on temperature and pressure. When a substance transforms from one state to another, this process is called a change of state. These are physical changes, not chemical ones. The substance itself remains the same; only the arrangement and motion of its particles changes.
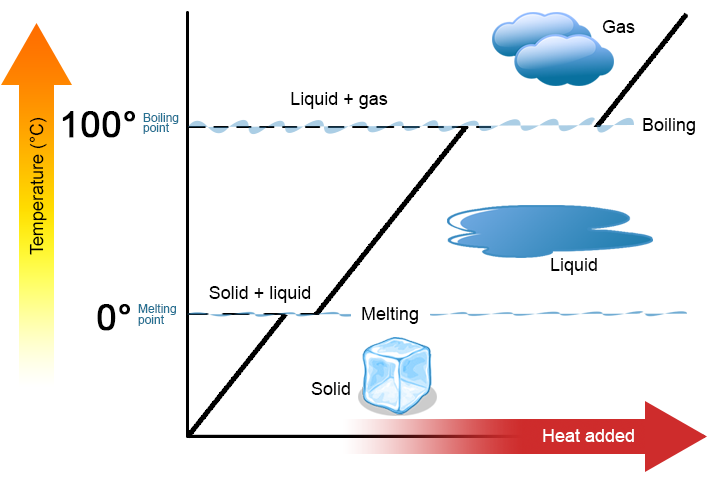
Changes of state involve either the gain or loss of energy, usually in the form of thermal energy. This energy affects the movement of particles and the forces between them. During a state change, the temperature of the substance remains constant while energy is used to break or form intermolecular forces.
Particle Explanation of State Changes
Melting (solid → liquid):

Particles gain energy, vibrate more, and overcome some of the strong forces holding them in fixed positions. The regular structure breaks down, and the substance becomes a liquid.
Freezing (liquid → solid):

Particles lose energy and move less. The attractive forces pull particles into fixed positions to form a solid structure.
Boiling (liquid → gas):

Particles gain enough energy to completely break the intermolecular bonds and escape the liquid. This happens at the boiling point.
Evaporation (liquid → gas):

Like boiling, but it only occurs at the surface of the liquid and below the boiling point.
Condensation (gas → liquid):

Particles lose energy and move closer together, forming a liquid.
Energy Transfer in Changes of State
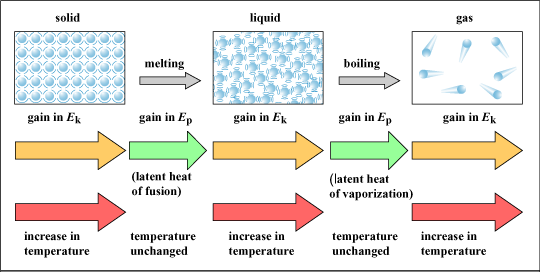
- Melting, boiling, and evaporation require thermal energy input – this energy increases the kinetic energy of particles.
- Freezing and condensation involve thermal energy release – particles lose kinetic energy and move closer together.
- During the change itself, temperature remains constant because energy is used to overcome or form intermolecular forces, not to increase kinetic energy.
| Change | Process Name | Energy Transfer | Effect on Particles |
|---|---|---|---|
| Solid → Liquid | Melting | Heat absorbed | Particles vibrate faster and break out of fixed positions |
| Liquid → Solid | Freezing | Heat released | Particles lose energy and lock into fixed positions |
| Liquid → Gas | Boiling (or Evaporation) | Heat absorbed | Particles move freely and spread apart |
| Gas → Liquid | Condensation | Heat released | Particles lose energy and come closer together |
Important Notes for Exams
- Melting and freezing happen at the same fixed temperature – the melting/freezing point.
- Boiling and condensation happen at the same fixed temperature – the boiling/condensation point.
- Evaporation occurs at all temperatures but only from the surface of a liquid.
- Changes of state are reversible and involve no change in chemical composition.
Example:
A block of ice is heated at a constant rate. Its temperature rises from -10 °C to 0 °C, then remains constant for a few minutes while the ice melts completely. After that, the temperature of the water rises steadily.
Explain why the temperature remains constant during the melting, even though heating continues.
▶️ Answer/Explanation
During melting, all the heat energy supplied is used to break the strong intermolecular forces between ice particles rather than increase their kinetic energy.
This process converts the solid to liquid without raising the temperature. The temperature stays constant until all the ice has melted.
Example:
The graph below shows how the temperature of a substance changes with time as it is heated at a constant rate:
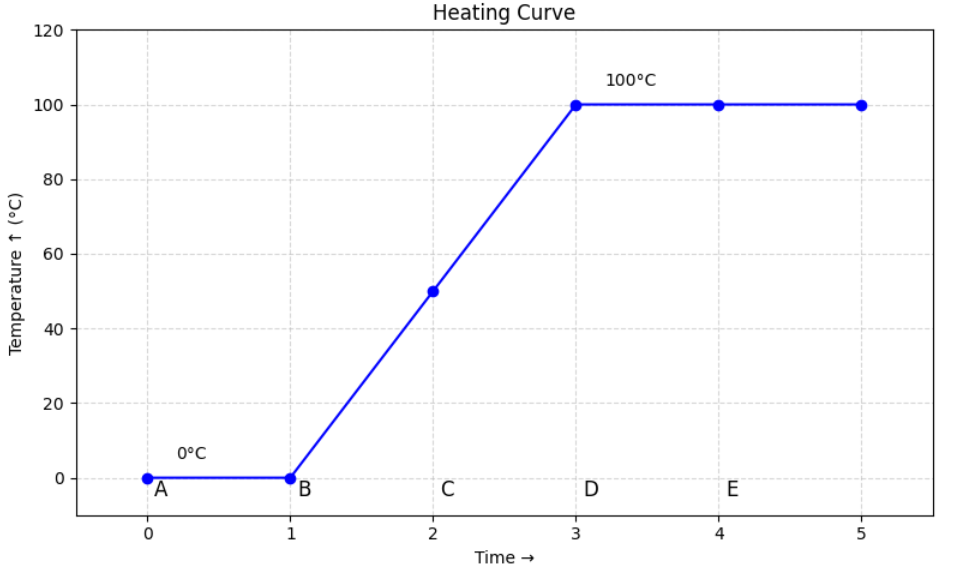
(a) Identify the sections where the substance is changing state.
(b) Explain why the temperature does not change during these sections even though heating continues.
▶️ Answer/Explanation
(a) The flat sections (B-C and D-E) represent the changes of state.
- B–C: melting (solid to liquid)
- D–E: boiling (liquid to gas)
(b) During these sections, the thermal energy is used to overcome intermolecular forces rather than increase kinetic energy. Hence, temperature remains constant until the state change is complete.
Example:
The diagram shows the change of phase of a substance on a temperature–time graph as it is heated at a constant rate.
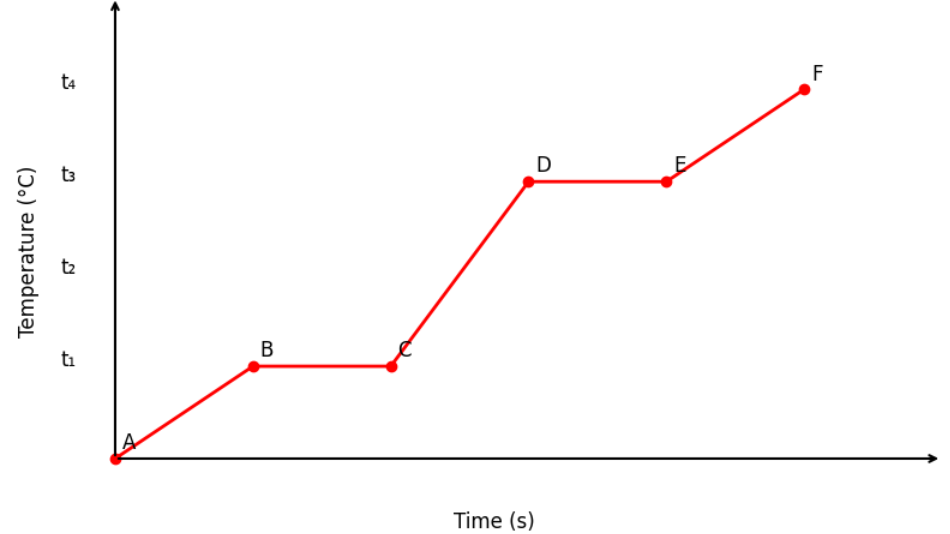
Answer the following:
- a. What do the parts AB, BC, CD, and DE represent?
- b. What is the melting point of the substance?
- c. What is the boiling point of the substance?
▶️ Answer/Explanation
a.
- AB: Rise in temperature of solid from 0°C to \( t_1^\circ \text{C} \)
- BC: Phase change — melting at constant temperature \( t_1^\circ \text{C} \)
- CD: Rise in temperature of liquid from \( t_1^\circ \text{C} \) to \( t_3^\circ \text{C} \)
- DE: Phase change — boiling at constant temperature \( t_3^\circ \text{C} \)
b.
Melting point of the substance = \(\boxed{t_1^\circ \text{C}}\)
c.
Boiling point of the substance = \(\boxed{t_3^\circ \text{C}}\)
