CIE IGCSE Physics (0625) The Atom Study Notes - New Syllabus
CIE IGCSE Physics (0625) The Atom Study Notes
LEARNING OBJECTIVE
- Understanding the concepts of The Atom
Key Concepts:
- Structure of an Atom
- Formation of Ions
- Alpha Particle Scattering and the Nuclear Model
Structure of an Atom
Structure of an Atom
An atom is the basic building block of matter.
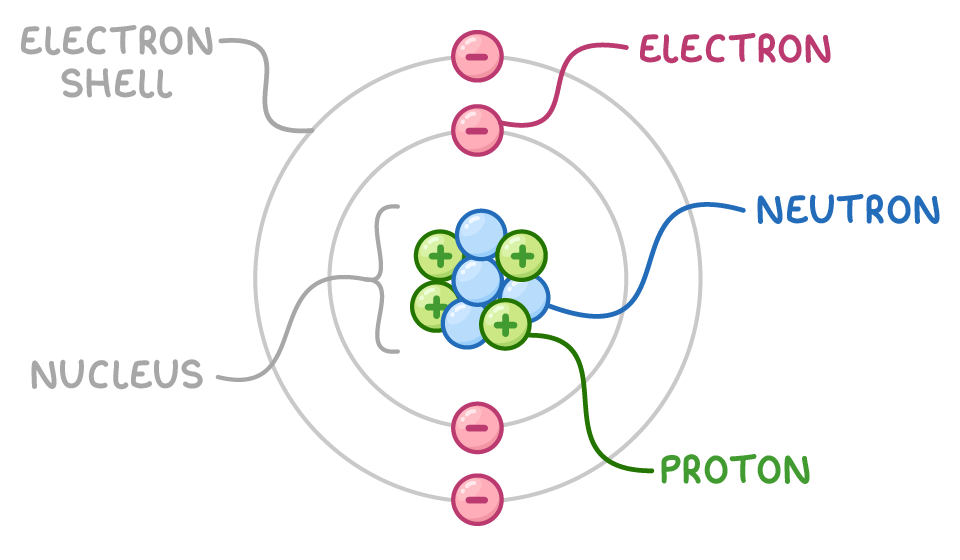
It consists of two main parts:
- Nucleus:
- Very small and dense central part of the atom.
- Contains protons and neutrons.
- Protons have a positive charge \((+1)\).
- Neutrons have no charge (neutral).
- Therefore, the nucleus is positively charged.
- Electrons:
- Very small, negatively charged particles \((-1)\).
- Move in orbits or energy levels (also called shells) around the nucleus.
- Electrons are arranged in specific shells depending on their energy.
Overall, an atom is electrically neutral because it has an equal number of protons and electrons.
| Particle | Charge | Location |
|---|---|---|
| Proton | +1 | Nucleus |
| Neutron | 0 (neutral) | Nucleus |
| Electron | -1 | Orbiting nucleus |
Example:
An atom of nitrogen has 7 protons, 7 neutrons, and 7 electrons. Describe the structure of this atom, including the charges and locations of its subatomic particles. Is the atom electrically neutral or charged?
▶️ Answer/Explanation
Step 1: Protons
Nitrogen has 7 protons, each with a charge of \( +1 \). They are located in the nucleus. Total positive charge = \( +7 \).
Step 2: Neutrons
7 neutrons are also in the nucleus. They have no charge, but contribute to the atom’s mass.
Step 3: Electrons
7 electrons are arranged in orbits (energy levels) around the nucleus. Each has a charge of \( -1 \), giving a total negative charge of \( -7 \).
Step 4: Overall Charge
Since there are equal numbers of protons and electrons (7 each), the atom is electrically neutral.
The nitrogen atom has a positively charged nucleus (protons and neutrons), and negatively charged electrons orbiting around it. The total charge is zero, so the atom is neutral.
Formation of Ions
Formation of Ions
An ion is an atom (or group of atoms) that has gained or lost one or more electrons. Ions are electrically charged because the number of protons and electrons are no longer equal.

Positive Ions (Cations)
- Formed when an atom loses electrons.
- There are now more protons than electrons, so the ion becomes positively charged.
- Usually formed by metals.
Example:

Sodium atom: \( \text{Na} \) → loses 1 electron → \( \text{Na}^+ \)
Negative Ions (Anions)
- Formed when an atom gains electrons.
- There are now more electrons than protons, so the ion becomes negatively charged.
- Usually formed by non-metals.
Example:
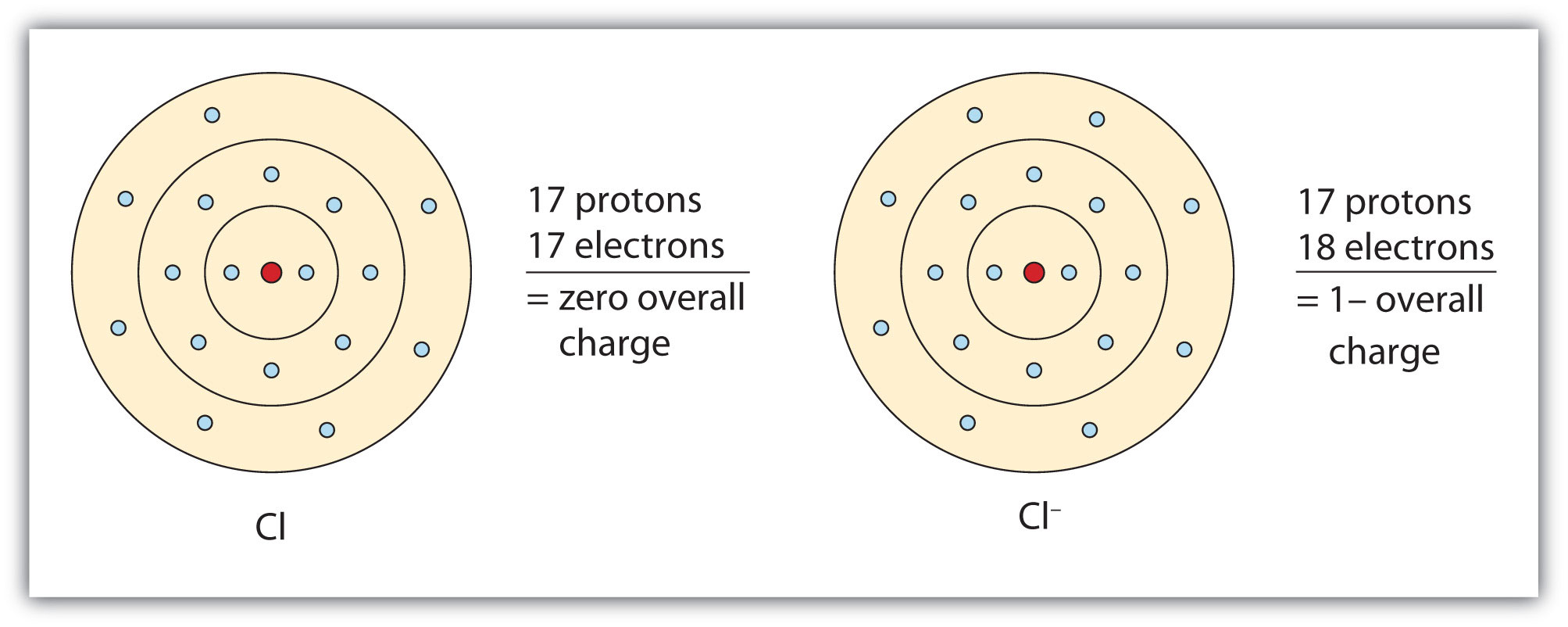
Chlorine atom: \( \text{Cl} \) → gains 1 electron → \( \text{Cl}^- \)
| Ion Type | Electron Change | Charge | Example |
|---|---|---|---|
| Positive Ion (Cation) | Loses electrons | Positive (+) | Na → Na⁺ |
| Negative Ion (Anion) | Gains electrons | Negative (−) | Cl → Cl⁻ |
Example:
An atom of calcium has 20 protons and 20 electrons. It loses 2 electrons to form an ion. What is the charge of the ion and why? Also, explain what happens when an atom of oxygen gains 2 electrons.
▶️ Answer/Explanation
1. Calcium Atom:
Initially: 20 protons (+) and 20 electrons (−) → atom is neutral.
Loses 2 electrons → now has 20 protons and 18 electrons.
Net charge = +2, so the ion formed is \( \text{Ca}^{2+} \)
2. Oxygen Atom:
Initially: 8 protons and 8 electrons → atom is neutral.
Gains 2 electrons → now has 10 electrons.
Net charge = −2, so the ion formed is \( \text{O}^{2-} \)
Conclusion:
- Calcium forms a positive ion (cation) by losing electrons.
- Oxygen forms a negative ion (anion) by gaining electrons.
Alpha Particle Scattering and the Nuclear Model
Alpha Particle Scattering and the Nuclear Model
The experiment was conducted by Rutherford and his team.A narrow beam of alpha particles (α) was directed at a thin sheet of gold foil.
- A detector was placed around the foil to observe the paths of the alpha particles.
Observations:
- Most alpha particles passed straight through the foil without any deflection.
- A small number of particles were deflected at small angles.
- Very few particles (about 1 in 8000) were deflected back at angles greater than 90°.

Conclusions & How They Support the Nuclear Model:
(a) A very small nucleus surrounded by mostly empty space:
- Since most alpha particles passed through the foil without deflection, atoms must be mostly empty space.
- The occasional deflection suggests that all the positive charge and mass are concentrated in a tiny central nucleus.
(b) A nucleus containing most of the mass of the atom:
- Some alpha particles were deflected at large angles or even bounced back.
- This would only be possible if the alpha particles collided with something very massive inside the atom.
- This showed that the nucleus contains almost all the mass of the atom.
(c) A nucleus that is positively charged:
- Alpha particles are positively charged.
- When they approached the nucleus, they were repelled rather than attracted.
- This showed that the nucleus is also positively charged, causing repulsion between like charges.
Example:
In Rutherford’s experiment, most alpha particles passed through the gold foil undeflected, but a few were deflected at large angles or bounced back. What does this tell us about the structure and charge of the atom?
▶️ Answer/Explanation
1. Most particles pass through:
This tells us that atoms are mostly empty space, allowing the alpha particles to pass through without interaction.
2. A few particles deflect or bounce back:
This suggests that the atom contains a small, dense, central nucleus where most of the mass is concentrated.
The fact that some particles bounce back indicates that this nucleus is also positively charged, since like charges (alpha particles and protons) repel each other.
Conclusion:
The experiment supports the nuclear model: atoms consist of a tiny, dense, positively charged nucleus surrounded by mostly empty space with electrons orbiting it.
