Edexcel iGCSE Physics -5.14P Core Practical: Specific Heat Capacity- Study Notes- New Syllabus
Edexcel iGCSE Physics -5.14P Core Practical: Specific Heat Capacity- Study Notes- New syllabus
Edexcel iGCSE Physics -5.14P Core Practical: Specific Heat Capacity- Study Notes -Edexcel iGCSE Physics – per latest Syllabus.
Key Concepts:
5.14P practical: investigate the specific heat capacity of materials including water and some solids
Practical: Investigating Specific Heat Capacity of Materials
This practical determines the specific heat capacity of different materials by measuring the energy supplied and the resulting temperature change.
Aim
To determine the specific heat capacity of water and solid materials.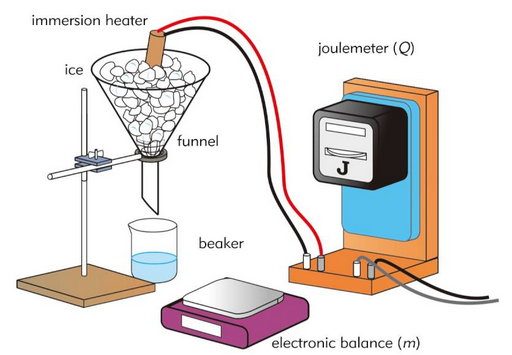
Apparatus
- Electric immersion heater
- Power supply
- Ammeter and voltmeter (or joulemeter)
- Thermometer or temperature probe
- Stopwatch
- Insulated container (for water)
- Solid metal block with heater hole
- Balance
Key Equation Used
\( \mathrm{\Delta E = m c \Delta T} \)
- \( \mathrm{\Delta E} \) = change in thermal energy (J)
- \( \mathrm{m} \) = mass (kg)
- \( \mathrm{c} \) = specific heat capacity (J/kg °C)
- \( \mathrm{\Delta T} \) = temperature change (°C)
Electrical energy supplied:
\( \mathrm{E = V I t} \)
Method A: Water
- Measure the mass of the water using a balance.
- Place the immersion heater and thermometer into the water.
- Record the initial temperature.
- Switch on the heater and start the stopwatch.
- Measure voltage and current.
- After a fixed time, record the final temperature.
- Calculate the energy supplied and specific heat capacity.
Method B: Solid Material
- Measure the mass of the metal block.
- Insert the heater and thermometer into the block.
- Insulate the block to reduce heat loss.
- Record the initial temperature.
- Switch on the heater for a measured time.
- Record the final temperature.
- Calculate specific heat capacity.
Variables
- Independent: material used
- Dependent: temperature change
- Controlled: heating time, power supplied, insulation
Conclusion
- Different materials have different specific heat capacities.
- Water has a much higher specific heat capacity than metals.
- More energy is needed to heat substances with higher specific heat capacity.
Safety Precautions
- Do not touch hot heaters or metal blocks.
- Keep water away from electrical connections.
- Switch off power before adjustments.
Sources of Error & Improvements
- Heat loss to surroundings → improve insulation.
- Temperature measurement lag → use digital probe.
- Energy lost in heater leads → include correction or reduce exposure.
Key Idea
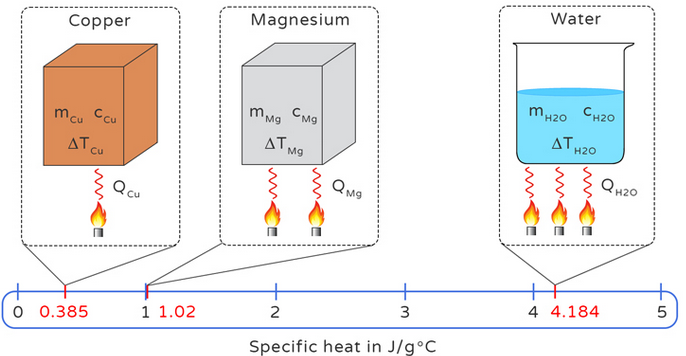
- Specific heat capacity depends on material.
- Electrical energy can be converted to thermal energy.
- Energy conservation applies.
Example
An immersion heater supplies \( \mathrm{2400\ J} \) of energy to \( \mathrm{0.50\ kg} \) of water.
The temperature rises by \( \mathrm{1.15^\circ C} \).
Calculate the specific heat capacity of water.
▶️ Answer / Explanation
Use:
\( \mathrm{c = \dfrac{\Delta E}{m \Delta T}} \)
\( \mathrm{c = \dfrac{2400}{0.50 \times 1.15}} \)
\( \mathrm{c \approx 4170\ J/kg\,^\circ C} \)
Example
A \( \mathrm{1.2\ kg} \) metal block absorbs \( \mathrm{3000\ J} \) of energy.
Its temperature rises by \( \mathrm{5.0^\circ C} \).
Calculate the specific heat capacity of the metal.
▶️ Answer / Explanation
Use:
\( \mathrm{c = \dfrac{3000}{1.2 \times 5.0}} \)
\( \mathrm{c = 500\ J/kg\,^\circ C} \)
