Edexcel iGCSE Physics -7.2–7.3 Atomic Structure, Nuclear Symbols, and Isotopes- Study Notes- New Syllabus
Edexcel iGCSE Physics -7.2–7.3 Atomic Structure, Nuclear Symbols, and Isotopes- Study Notes- New syllabus
Edexcel iGCSE Physics -7.2–7.3 Atomic Structure, Nuclear Symbols, and Isotopes- Study Notes -Edexcel iGCSE Physics – per latest Syllabus.
Key Concepts:
7.2 describe the structure of an atom in terms of protons, neutrons and electrons and use symbols such as ¹⁴₆C to describe particular nuclei
7.3 know the terms atomic (proton) number, mass (nucleon) number and isotope
Structure of the Atom and Nuclear Notation
An atom consists of a very small, dense nucleus surrounded by electrons. Understanding the structure of the atom and the symbols used to represent nuclei is essential in radioactivity and nuclear physics.
Basic Structure of an Atom
An atom is made up of three types of subatomic particles: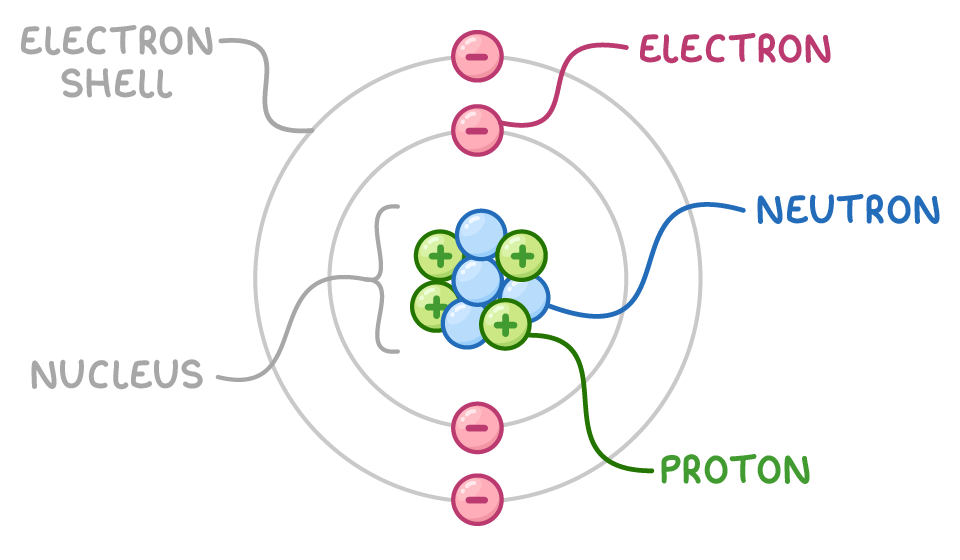
- Protons
- Neutrons
- Electrons
Arrangement:
- The nucleus contains protons and neutrons.
- Electrons move around the nucleus in shells.
- Most of the atom is empty space.
Properties of Subatomic Particles
- Proton: positive charge, found in the nucleus.
- Neutron: no charge, found in the nucleus.
- Electron: negative charge, moves around the nucleus.
Key idea: In a neutral atom, the number of protons equals the number of electrons.
Atomic Number and Mass Number
Atomic number (Z):
- Number of protons in the nucleus.
- Determines the element.
Mass number (A):
- Total number of protons and neutrons.
\( \mathrm{A = \text{number of protons} + \text{number of neutrons}} \)
Nuclear Notation
Nuclei are represented using the following symbol:
\( \mathrm{^{A}_{Z}X} \)
- \( \mathrm{X} \) = chemical symbol of the element
- \( \mathrm{Z} \) = atomic number (protons)
- \( \mathrm{A} \) = mass number (protons + neutrons)
Example:
\( \mathrm{^{14}_{6}C} \)
- Protons = 6
- Neutrons = \( \mathrm{14 – 6 = 8} \)
- Electrons (neutral atom) = 6
Key Points About Nuclei
- The nucleus contains nearly all the mass of the atom.
- Electrons have very small mass.
- Nuclear changes involve protons and neutrons, not electrons.
Example
An atom is represented by the symbol \( \mathrm{^{23}_{11}Na} \). Determine the number of protons, neutrons, and electrons in a neutral atom.
▶️ Answer / Explanation
- Protons = atomic number = 11
- Neutrons = \( \mathrm{23 – 11 = 12} \)
- Electrons = 11 (neutral atom)
Example
A nucleus contains 17 protons and 18 neutrons. Write the nuclear symbol for this nucleus and state the number of electrons in the neutral atom.
▶️ Answer / Explanation
Mass number:
\( \mathrm{A = 17 + 18 = 35} \)
Nuclear symbol:
\( \mathrm{^{35}_{17}Cl} \)
- Electrons in a neutral atom = 17
Atomic Number, Mass Number and Isotopes
Atoms and nuclei are described using specific terms that identify the type of element and the composition of its nucleus. The key terms are atomic (proton) number, mass (nucleon) number, and isotope.
Atomic (Proton) Number
Definition: The atomic number is the number of protons in the nucleus of an atom.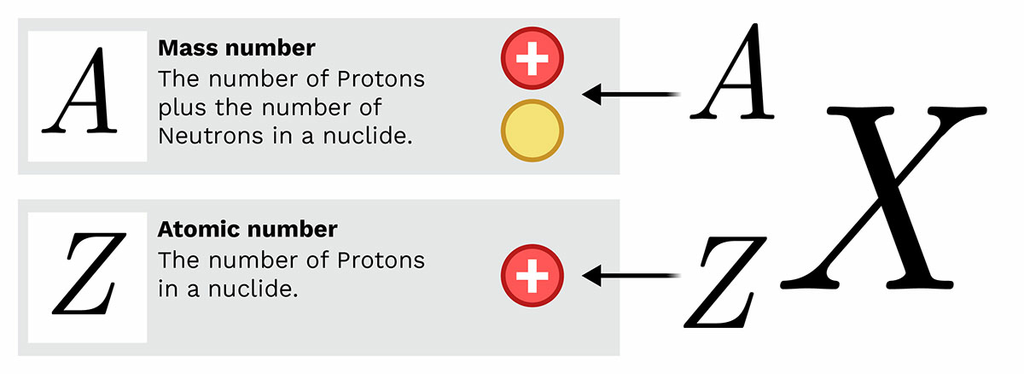
Symbol: \( \mathrm{Z} \)
Key points:
- Determines the element.
- All atoms of the same element have the same atomic number.
- In a neutral atom, atomic number = number of electrons.
Example idea: Any atom with \( \mathrm{Z = 6} \) is carbon.
Mass (Nucleon) Number
Definition: The mass number is the total number of protons and neutrons in the nucleus.
Symbol: \( \mathrm{A} \)
Relationship:
\( \mathrm{A = \text{number of protons} + \text{number of neutrons}} \)
Key points:
- Mass number can vary for atoms of the same element.
- Most of an atom’s mass comes from the nucleus.
Isotopes
Definition: Isotopes are atoms of the same element that have the same atomic number but different mass numbers.
Meaning:
- Same number of protons.
- Different number of neutrons.
- Same chemical properties.
- Different nuclear properties.
Key idea: Isotopes differ only in the number of neutrons.
Nuclear Notation Reminder
Nuclei are written in the form:
\( \mathrm{^{A}_{Z}X} \)
- \( \mathrm{Z} \) = atomic (proton) number
- \( \mathrm{A} \) = mass (nucleon) number
Example:
\( \mathrm{^{14}_{6}C} \)
- Protons = 6
- Neutrons = \( \mathrm{14 – 6 = 8} \)
Example
Two nuclei are \( \mathrm{^{37}_{17}Cl} \) and \( \mathrm{^{35}_{17}Cl} \). Explain why these nuclei are isotopes and state one way in which they are different.
▶️ Answer / Explanation
- Both have atomic number 17.
- They are atoms of the same element, chlorine.
- The mass numbers are different.
- The number of neutrons is different.
Example
A nucleus has a mass number of 56 and contains 26 protons. Determine the atomic number, number of neutrons, and state whether another nucleus with 26 protons and mass number 54 is an isotope.
▶️ Answer / Explanation
Atomic number:
\( \mathrm{Z = 26} \)
Neutrons:
\( \mathrm{56 – 26 = 30} \)
- The second nucleus has the same atomic number.
- It has a different mass number.
- Therefore, it is an isotope of the same element.
