Edexcel iGCSE Physics -7.4–7.5 Types and Properties of Alpha, Beta, and Gamma Radiation- Study Notes- New Syllabus
Edexcel iGCSE Physics -7.4–7.5 Types and Properties of Alpha, Beta, and Gamma Radiation- Study Notes- New syllabus
Edexcel iGCSE Physics -7.4–7.5 Types and Properties of Alpha, Beta, and Gamma Radiation- Study Notes -Edexcel iGCSE Physics – per latest Syllabus.
Key Concepts:
7.4 know that alpha (α) particles, beta (β⁻) particles, and gamma (γ) rays are ionising radiations emitted from unstable nuclei in a random process
7.5 describe the nature of alpha (α) particles, beta (β⁻) particles and gamma (γ) rays, and recall that they may be distinguished in terms of penetrating power and ability to ionise
Ionising Radiations from Unstable Nuclei
Some atomic nuclei are unstable. These nuclei emit radiation in order to become more stable. The three main types of nuclear radiation are alpha (α) particles, beta (β⁻) particles, and gamma (γ) rays. All three are ionising and are emitted in a random process.
Key Statement
Statement: Alpha particles, beta particles, and gamma rays are ionising radiations emitted from unstable nuclei in a random process.
Key idea: It is impossible to predict when a particular nucleus will decay.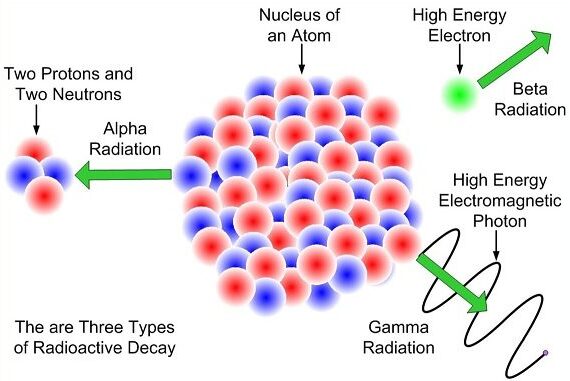
Alpha (α) Particles
Nature:
- Consist of two protons and two neutrons.
- Same as a helium nucleus.
Properties:
- Strongly ionising.
- Short range in air.
- Emitted from heavy unstable nuclei.
Beta (β⁻) Particles
Nature:
- Fast-moving electrons.
- Produced when a neutron changes into a proton.
Properties:
- Moderately ionising.
- Longer range than alpha particles.
- Emitted from unstable nuclei with too many neutrons.
Gamma (γ) Rays
Nature:
- Electromagnetic waves.
- No mass and no charge.
Properties:
- Weakly ionising.
- Very penetrating.
- Often emitted after alpha or beta decay.
Ionising Nature of Nuclear Radiation
Ionisation: Ionisation occurs when radiation removes electrons from atoms, forming ions.
- Alpha → most ionising
- Beta → moderately ionising
- Gamma → least ionising
Key idea: Greater ionisation usually means lower penetrating power.
Random Nature of Radioactive Decay
- Decay occurs spontaneously.
- It cannot be predicted when a nucleus will decay.
- External factors (temperature, pressure) do not affect decay.
Important: Random does not mean unpredictable overall – large samples decay at a steady average rate.
Example
Explain why radioactive decay is described as a random process even though the activity of a radioactive source decreases smoothly over time.
▶️ Answer / Explanation
- It is impossible to predict when a single nucleus will decay.
- Each nucleus decays independently.
- With many nuclei, an average rate of decay is observed.
- This produces a smooth decrease in activity.
Example
A radioactive source emits both beta particles and gamma rays. Explain how their ionising abilities differ and relate this to their properties.
▶️ Answer / Explanation
- Beta particles are charged and have mass.
- They cause moderate ionisation.
- Gamma rays have no charge or mass.
- They interact less with atoms and are weakly ionising.
Nature of Alpha, Beta and Gamma Radiation
Unstable nuclei emit alpha (α) particles, beta (β⁻) particles, and gamma (γ) rays. These radiations differ in their nature, ability to ionise, and penetrating power.
Alpha (α) Particles![]()
Nature:
- Consist of two protons and two neutrons.
- Same as a helium nucleus.
- Have a positive charge.
Ionising ability:
- Very strongly ionising.
- Cause many ionisations over a short distance.
Penetrating power:
- Very low penetrating power.
- Stopped by paper or a few centimetres of air.
Beta (β⁻) Particles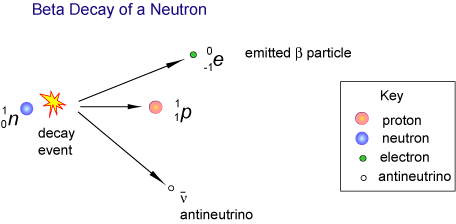
Nature:
- Fast-moving electrons.
- Have a negative charge.
- Much smaller mass than alpha particles.
Ionising ability:
- Moderately ionising.
- Cause fewer ionisations than alpha particles.
Penetrating power:
- Moderate penetrating power.
- Stopped by a few millimetres of aluminium.
Gamma (γ) Rays
Nature:
- Electromagnetic waves.
- No mass and no charge.
- Travel at the speed of light.
Ionising ability:
- Weakly ionising.
- Cause very few ionisations.
Penetrating power:
- Very high penetrating power.
- Reduced by thick lead or concrete.
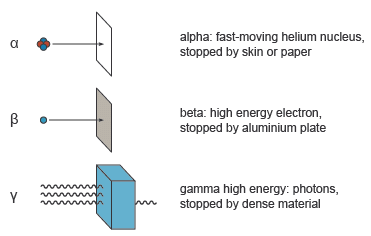
Comparing Ionising Ability and Penetrating Power
- Alpha: most ionising, least penetrating.
- Beta: moderate ionising and penetration.
- Gamma: least ionising, most penetrating.
Key idea: There is an inverse relationship between ionising ability and penetrating power.
Example
A radioactive source is placed near a detector. The count rate drops to zero when a sheet of paper is placed between the source and detector. Identify the radiation and explain your answer.
▶️ Answer / Explanation
- Paper stops alpha particles.
- Alpha radiation has very low penetrating power.
- The radiation must be alpha.
- Beta and gamma would pass through paper.
Example
Two radiations pass through paper, but only one passes through a thin sheet of aluminium. Identify both radiations and compare their ionising abilities.
▶️ Answer / Explanation
- Beta passes through paper but is stopped by aluminium.
- Gamma passes through both paper and aluminium.
- Beta is more ionising than gamma.
- Gamma is weakly ionising but highly penetrating.
