Edexcel A Level (IAL) Biology -1.1 The Importance of Water- Study Notes- New Syllabus
Edexcel A Level (IAL) Biology -1.1 The Importance of Water- Study Notes- New syllabus
Edexcel A Level (IAL) Biology -1.1 The Importance of Water- Study Notes -Edexcel A level Biology – per latest Syllabus.
Key Concepts:
- Understand the importance of water as a solvent in transport, including its dipole nature
Importance of Water as a Solvent in Transport (Dipole Nature)
🌱 Introduction
- Water is one of the most important biological molecules on Earth.
- It acts as a universal solvent and is vital for the transport of substances in living organisms.
- Because of its dipole (polar) nature, water can dissolve and carry many essential substances like ions, gases, and nutrients.
🚚 Transport of Substances in Living Organisms
Living organisms need to exchange and transport essential materials such as:
- Oxygen and carbon dioxide
- Nutrients and hormones
- Waste products
In small organisms: Diffusion alone is enough due to a large surface area to volume ratio.
In larger organisms:
Surface area to volume ratio decreases → diffusion distance increases → metabolic rate increases.
Hence, diffusion becomes too slow, and they develop a mass transport system.
⚙️ Features of a Mass Transport System
| Feature | Description |
|---|---|
| 1. Transport network | System of tubes (e.g., blood vessels, xylem) to move substances. |
| 2. Transport medium | Fluid like blood, lymph, or sap that carries materials. |
| 3. Controlled direction | Flow maintained in one direction (valves, pressure gradient). |
| 4. Maintenance of speed | Heart contractions or pressure differences keep flow efficient. |
💧 The Structure & Dipole Nature of Water
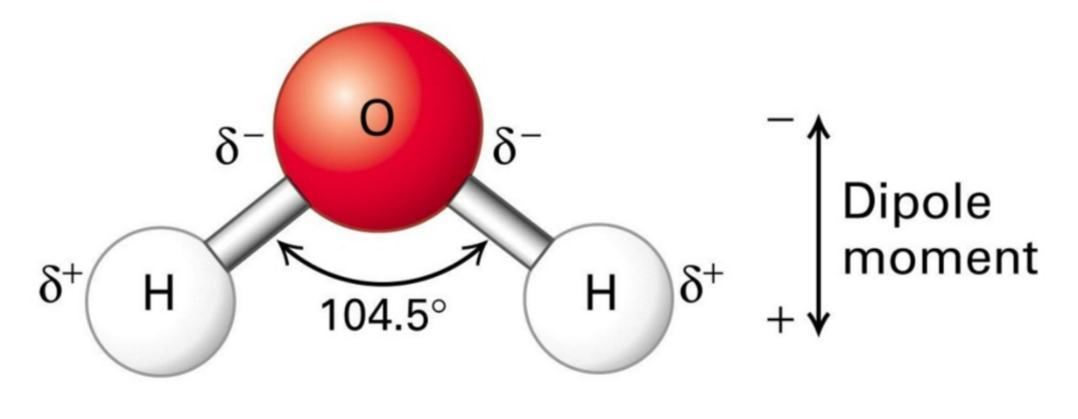
- Water molecule (H₂O) is made up of two hydrogen atoms and one oxygen atom.
- Due to uneven charge distribution:
- Oxygen → slightly negative (δ–) Hydrogen → slightly positive (δ+).
- This makes water a polar molecule (dipole) – one end positive, one end negative.
- Water molecules form hydrogen bonds with each other – weak individually but strong collectively.
🧬 Water as a Solvent
Because of its polarity, water can dissolve many substances, especially those that are ionic or polar.
- Mineral ions → Na⁺, K⁺, Cl⁻
- Organic molecules → glucose, amino acids
- Gases → O₂, CO₂ (to a limited extent)
📦 Biological Significance
| Example | Role of Water as Solvent |
|---|---|
| Blood plasma | Transports nutrients, hormones, and waste products. |
| Xylem & Phloem | Transport of minerals, sugars, and water in plants. |
| Cytoplasm | Medium for biochemical reactions within cells. |
🌿 Hydrogen Bonding & Cohesion
Hydrogen bonds create cohesion (water molecules stick together).
Adhesion occurs when water molecules stick to other surfaces.
Together, cohesion + adhesion enable capillary action – essential for upward water transport in xylem.
🌡️ Thermal Properties of Water
Water has a high specific heat capacity → absorbs much heat without large temperature change.
This helps to:
- Stabilize body temperature (homeostasis)
- Regulate environmental temperature for aquatic organisms
📊 Summary Table
| Property of Water | Biological Importance |
|---|---|
| Polar (Dipole) Nature | Dissolves ionic and polar molecules for transport. |
| Hydrogen Bonding | Cohesion & adhesion help in xylem water movement. |
| High Specific Heat Capacity | Maintains stable temperature in organisms. |
| Liquid at Room Temperature | Ideal for continuous flow and circulation. |
| Solvent for Biochemical Reactions | Enables metabolic reactions within cells. |
🧠 Quick Recap
Water = Polar molecule → forms hydrogen bonds
Acts as a solvent for transport of ions & molecules
Enables cohesion & adhesion → water movement in xylem
High heat capacity → temperature stability
Essential medium for mass transport (blood, xylem, phloem)
