Edexcel A Level (IAL) Biology -1.2 -1.5 Saccharides - Study Notes- New Syllabus
Edexcel A Level (IAL) Biology -1.2 -1.5 Saccharides- Study Notes -Edexcel A level Biology – per latest Syllabus.
Key Concepts:
- 1.2 (i) know the difference between monosaccharides, disaccharides and polysaccharides, including glycogen and starch (amylose and amylopectin)
- (ii) be able to relate the structures of monosaccharides, disaccharides and polysaccharides to their roles in providing and storing energy
- 1.3 Use a semi-quantitative method with Benedict’s reagent to estimate the concentrations of reducing sugars and with iodine solution to estimate the concentrations of starch, using colour standards.
- 1.4 know how monosaccharides (glucose, fructose and galactose) join together to form disaccharides (maltose, sucrose and lactose) and polysaccharides (glycogen, amylose and amylopectin) through condensation reactions forming glycosidic bonds, and how these can be split through hydrolysis reactions
- 1.5 (i) know how a triglyceride is synthesised by the formation of ester bonds during condensation reactions between glycerol and three fatty acids
- (ii) know the differences between saturated and unsaturated lipids
Understanding Monosaccharides, Disaccharides & Polysaccharides
📌 Introduction
Carbohydrates are organic molecules made of carbon (C), hydrogen (H), and oxygen (O) in the general formula Cₙ(H₂O)ₙ.
They are the main source of energy for living organisms and also act as energy storage molecules in both plants and animals.
🍯 (i) Difference Between Monosaccharides, Disaccharides & Polysaccharides
| Type | Number of Sugar Units | Example | Solubility | Function |
|---|---|---|---|---|
| Monosaccharide | 1 (single unit) | Glucose, Fructose, Galactose | Soluble | Quick energy source |
| Disaccharide | 2 units (formed by condensation of 2 monosaccharides) | Maltose, Sucrose, Lactose | Soluble | Easily transported form of sugar |
| Polysaccharide | Many monosaccharides joined together | Starch (amylose + amylopectin), Glycogen | Insoluble | Long-term energy storage |
🧩 Monosaccharides – Simple Sugars
Structure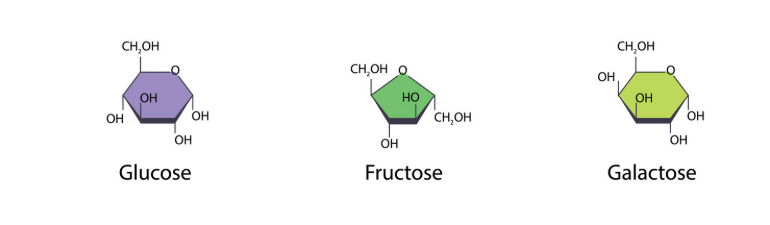
- Smallest carbohydrate molecules (single sugar units).
- Usually have the formula C₆H₁₂O₆.
- Contain hydroxyl (-OH) groups → polar → dissolve easily in water.
Function
- Main respiratory substrates (especially glucose).
- Provide instant energy as they can be broken down quickly during respiration.
Examples
| Monosaccharide | Found In | Key Role |
|---|---|---|
| Glucose | Blood, plant sap | Main source of energy |
| Fructose | Fruits, honey | Sweetest natural sugar |
| Galactose | Milk (as part of lactose) | Component of milk sugar |
🍭 Disaccharides – Double Sugars
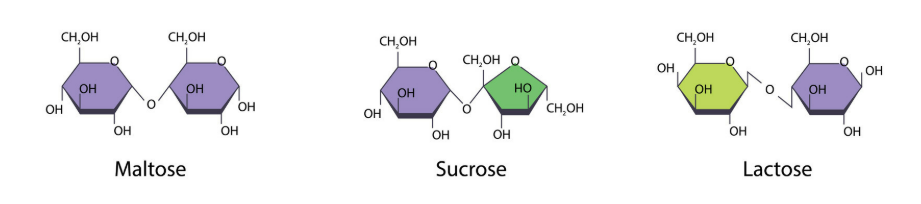
Structure
- Formed by condensation (joining of two monosaccharides) → releases one molecule of water (H₂O).
- Joined by a glycosidic bond.
- Can be broken down by hydrolysis back into monosaccharides.
Examples
| Disaccharide | Monosaccharides Joined | Found In | Function |
|---|---|---|---|
| Maltose | Glucose + Glucose | Germinating seeds | Energy source for growth |
| Sucrose | Glucose + Fructose | Sugar cane, plant transport sugar | Transported in phloem |
| Lactose | Glucose + Galactose | Milk | Energy for infants |
🍞 Polysaccharides – Complex Carbohydrates
Structure
- Formed by joining many glucose units through glycosidic bonds.
- Insoluble, so do not affect osmotic balance.
- Suitable for long-term energy storage.
🌾 Starch (Plant Storage Carbohydrate)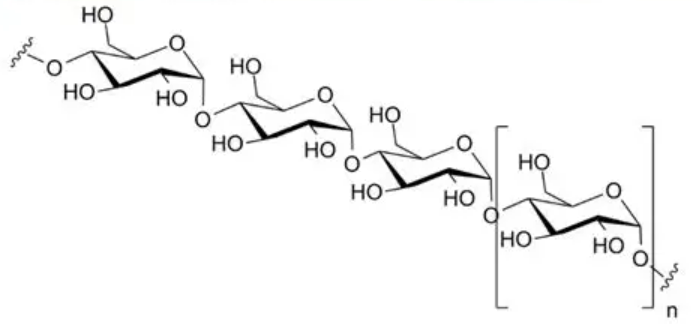
Starch is made up of two types of glucose polymers:
- Amylose
Long, unbranched chain of α-glucose.
Joined by 1,4-glycosidic bonds.
Coils into a compact helical shape → stores large amounts of glucose in a small space. - Amylopectin
Branched chain of α-glucose.
Has both 1,4- and 1,6-glycosidic bonds.
Branching allows enzymes to break glucose off quickly when needed for respiration.
Together, amylose + amylopectin form starch granules in plants (e.g. potatoes, seeds).
🧬 Glycogen (Animal Storage Carbohydrate)
- Made of α-glucose units, similar to amylopectin but more highly branched.
- Stored mainly in the liver and muscle cells.
- Branching helps in rapid glucose release during respiration for quick energy.
- Compact and insoluble, so ideal for storage without drawing water in by osmosis.
⚡ (ii) Relating Structure to Function
| Carbohydrate | Structural Features | Function / Role |
|---|---|---|
| Monosaccharides | Small, soluble molecules | Quick source of energy for respiration |
| Disaccharides | Two-unit sugars, soluble | Transportable form of sugar (e.g., sucrose in plants) |
| Amylose (Starch) | Unbranched helix, compact | Good for slow energy release and dense storage |
| Amylopectin (Starch) | Branched chains | Quick release of glucose when needed |
| Glycogen | Highly branched, compact | Rapid energy supply for animals |
🧠 Quick Recap
Mono = 1 → instant energy (e.g., glucose)
Di = 2 → transport form (e.g., sucrose, lactose)
Poly = many → storage (e.g., starch in plants, glycogen in animals)
Amylose → coiled, compact
Amylopectin → branched, quick energy
Glycogen → highly branched, stored in muscles/liver
All are made from α-glucose, not β-glucose (cellulose not part of this topic).
CORE PRACTICAL 1 – Semi-Quantitative Estimation of Reducing Sugars (Benedict’s) & Starch (Iodine) Using Colour Standards
🧠 Principle (Short)
- Benedict’s test: reducing sugars reduce Cu²⁺ → Cu₂O; colour changes from blue → green → yellow → orange → brick-red depending on sugar amount.
- Iodine test: iodine + starch → blue-black complex; intensity of blue-black indicates starch concentration.
- Semi-quantitative: prepare standards of known concentrations, test them side-by-side with unknowns under identical conditions, and compare colour to estimate unknown concentration (visually or using colorimeter).
📋 Overview of Procedure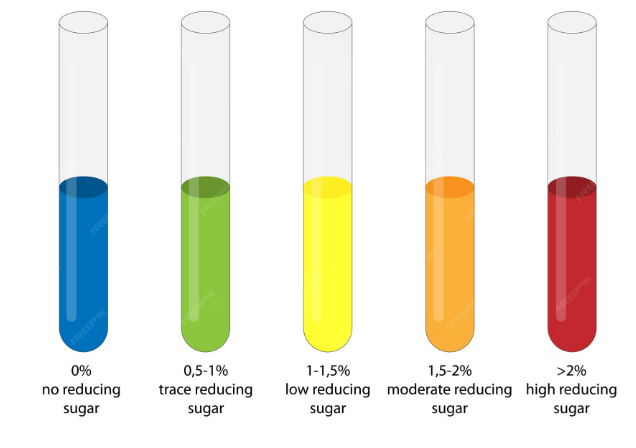
- Prepare standards for glucose (reducing sugar) and starch.
- Prepare samples (dilute if necessary).
- Run Benedict’s test on glucose standards and samples.
- Run Iodine test on starch standards and samples.
- Compare colours with standards and estimate concentrations.
- Repeat in triplicate and report mean ± range.
🧪 Materials & Reagents
- Benedict’s reagent (freshly prepared)
- Iodine solution (I₂ + KI)
- Standard glucose solution (e.g., 10 g dm⁻³)
- Standard soluble starch solution
- Distilled water, pipettes, test tubes, water bath (~95°C), white tile
- Labels, marker, measuring cylinder
- Optional: colorimeter or smartphone camera for colour comparison
🔧 Preparing Colour Standards
| Test | Concentration Range (g dm⁻³) | Preparation Tip |
|---|---|---|
| Reducing Sugar (Glucose) | 0, 2, 4, 6, 8, 10 | Prepare 10 g dm⁻³ stock → serially dilute to get 8, 6, 4, 2, 0. |
| Starch | 0, 0.5, 1.0, 1.5, 2.0, 2.5 | Prepare 2.5 g dm⁻³ stock and dilute accordingly. |
🧪 Benedict’s Test – Detailed Protocol
- Label test tubes: G0, G2, G4, G6, G8, G10 (standards), and S1, S2 (samples).
- Add 2.0 cm³ standard/sample + 2.0 cm³ Benedict’s reagent (same ratio for all).
- Place tubes in boiling-water bath (≈95°C) for 5 minutes – constant time for all tubes.
- Cool under running water for 1 min, stop reaction.
- Compare colours on white tile to standards → estimate concentration.
- If colour lies between standards → interpolate.
- Repeat triplicates → record mean ± SD.
Typical Benedict’s Colour Scale
| Approx. Conc. (g dm⁻³) | Observed Colour |
|---|---|
| 0 | Blue (no change) |
| 2 | Greenish-blue |
| 4 | Yellow |
| 6 | Orange |
| 8–10 | Brick-red precipitate |
🧪 Iodine Test – Detailed Protocol
- Label tubes: S0 (blank), S0.5, S1.0, S1.5, S2.0, S2.5 (standards) and sample tubes.
- Add 2.0 cm³ of each starch standard/sample + 2 drops iodine solution.
- Mix gently, compare immediately on white tile under same lighting.
- Match intensity of blue-black colour with closest standard → estimate concentration.
- Repeat in triplicate, average results.
🔁 Dilutions & Calculations
Actual concentration = measured conc. × dilution factor
Example: diluted 1:5, estimated 2 g dm⁻³ → actual = 2 × 5 = 10 g dm⁻³
✅ Controls & Good Practice
- Use blank (distilled water + reagent) and positive control (known glucose/starch).
- Run standards and unknowns in same batch.
- Use same reagent batch, equal volumes, fixed heating time.
- Compare under same lighting; perform triplicates.
⚠️ Safety & Disposal
- Benedict’s reagent (copper salts) → wear gloves & goggles.
- Iodine is toxic → avoid contact and fumes.
- Use heatproof gloves when handling hot tubes.
- Dispose of copper-containing waste properly.
🔎 Limitations & Common Pitfalls
- Benedict’s detects only reducing sugars (not sucrose unless hydrolysed).
- Vitamin C and other reducers can interfere.
- Iodine detects starch but not smaller dextrins.
- Colour comparison is subjective use colorimeter for accuracy (540 nm for Benedict’s, 620 nm for iodine starch).
- Ensure standards cover sample range avoid saturation at high conc.
💡Quick Recap
| Test | Key Reaction | Colour Change | Interpretation |
|---|---|---|---|
| Benedict’s | Cu²⁺ → Cu₂O (reduction) | Blue → Green → Yellow → Orange → Red | Increasing intensity = more reducing sugar |
| Iodine | I₂ + Starch → Blue-black complex | Brown → Blue-black | Darker colour = more starch |
| Accuracy Tip | Run standards & samples together, identical time/temp/volume, compare under same light. | ||
Formation & Breakdown of Carbohydrates
(Condensation & Hydrolysis – Glycosidic Bond Formation)
🌱 Introduction
Carbohydrates are built by joining smaller sugar units (monosaccharides) together to form disaccharides and polysaccharides.
This happens through condensation reactions, and the reverse (breaking apart) happens by hydrolysis reactions.
These reactions are central to how energy is stored and released in living organisms.
🍬 Monosaccharides – The Building Blocks
Examples: Glucose, Fructose, Galactose
- They are single sugar molecules (simple carbohydrates).
- Contain hydroxyl (–OH) groups → can react with other sugar molecules.
- Can link together to form larger carbohydrates.
🧩 Condensation Reactions – Building Carbohydrates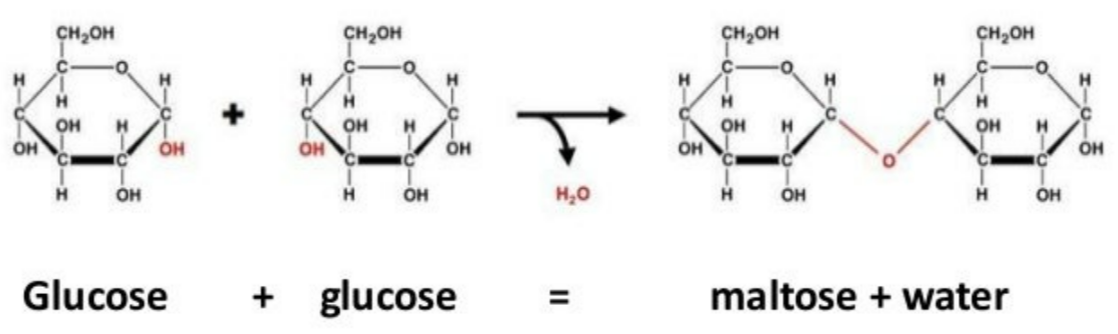
Definition
A condensation reaction is when two monosaccharide molecules join together, forming a glycosidic bond and releasing one molecule of water (H₂O).
🧪 What Happens Chemically
- The -OH group from one monosaccharide and a -H from another combine → H₂O is released.
- The remaining oxygen atom forms a glycosidic bond (C-O-C) between the two sugar molecules.
Bond formed: Glycosidic bond
Type of reaction: Condensation (water is removed)
🍭 Formation of Disaccharides
| Disaccharide | Formed From | Type of Bond | Reaction |
|---|---|---|---|
| Maltose | Glucose + Glucose | 1,4-glycosidic bond | Condensation |
| Sucrose | Glucose + Fructose | 1,2-glycosidic bond | Condensation |
| Lactose | Glucose + Galactose | 1,4-glycosidic bond | Condensation |
Enzyme example: Maltase breaks maltose → glucose (via hydrolysis)
🍞 Formation of Polysaccharides
When many monosaccharides join together through repeated condensation reactions, long chains form called polysaccharides.
Examples:
- Amylose → Long, unbranched chain of α-glucose (1,4 bonds)
- Amylopectin → Branched α-glucose chain (1,4 and 1,6 bonds)
- Glycogen → Highly branched α-glucose polymer (1,4 and 1,6 bonds)
All these polymers are made from α-glucose joined by glycosidic bonds.
💧 Hydrolysis Reactions – Breaking Down Carbohydrates
Definition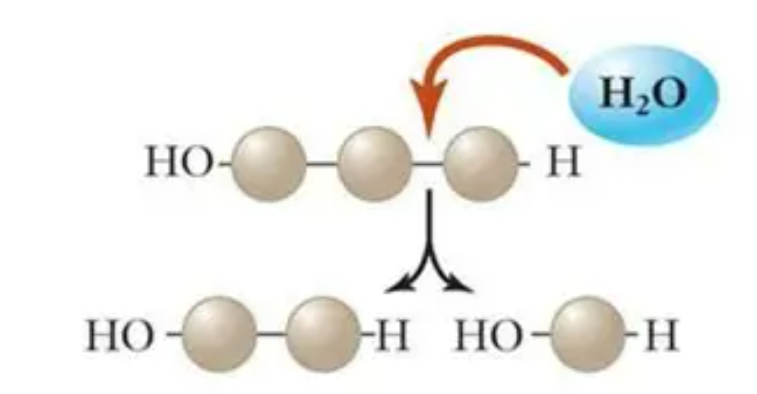
A hydrolysis reaction is the reverse of condensation – it breaks glycosidic bonds by adding water.
🧪 What Happens Chemically
- Water (H₂O) is added back, splitting the bond between two monosaccharides.
- Each molecule regains a hydroxyl (–OH) and a hydrogen (–H).
Bond broken: Glycosidic bond
Type of reaction: Hydrolysis (water is added)
🔁 Example of Hydrolysis
| Complex Sugar | Hydrolysed Into | Enzyme | Type of Reaction |
|---|---|---|---|
| Maltose | Glucose + Glucose | Maltase | Hydrolysis |
| Sucrose | Glucose + Fructose | Sucrase | Hydrolysis |
| Lactose | Glucose + Galactose | Lactase | Hydrolysis |
| Starch / Glycogen | Many Glucose Units | Amylase | Hydrolysis |
⚡ Why These Reactions Are Important
| Reaction Type | Function in Organisms |
|---|---|
| Condensation | Builds energy storage molecules (starch, glycogen) |
| Hydrolysis | Breaks down carbohydrates to release glucose for respiration |
| Reversibility | Allows flexibility between storage and energy supply |
🧠 Quick Recap
Condensation → joins monosaccharides → forms glycosidic bond + releases water
Hydrolysis → breaks glycosidic bond → adds water
📌 Polysaccharides:
Repeated condensation of α-glucose → amylose, amylopectin, glycogen
All bonds between sugars = glycosidic bonds
Formation & Types of Lipids
(Triglyceride Synthesis + Saturated vs Unsaturated Lipids)
🌱 Introduction
Lipids are large, energy-rich organic molecules made up of carbon (C), hydrogen (H), and oxygen (O) – but with much less oxygen than carbohydrates.
They are insoluble in water but soluble in organic solvents (like ethanol).
The most common lipids in living organisms are triglycerides – found in fats and oils.
🧩 (i) Formation of Triglycerides – Condensation Reaction
What is a Triglyceride?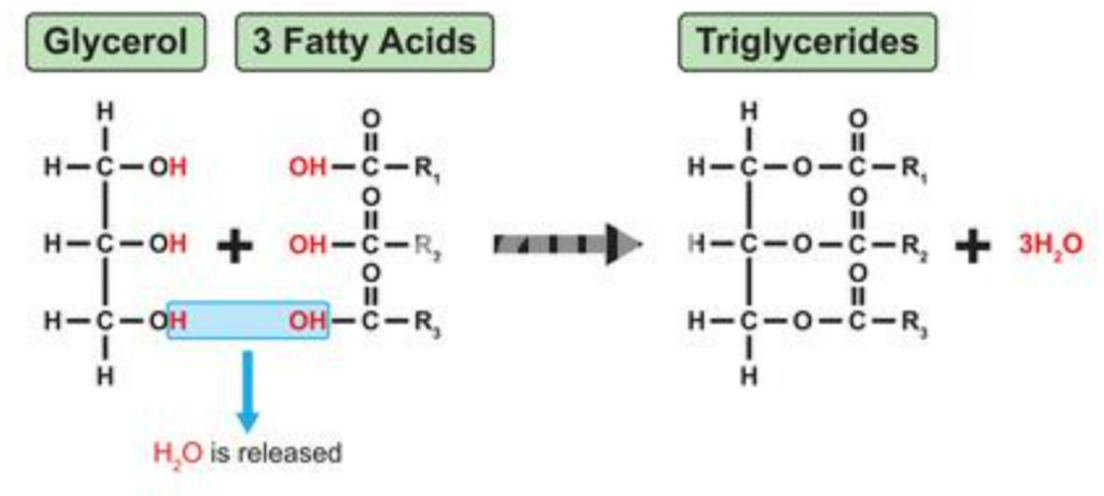
A triglyceride is formed when one molecule of glycerol joins with three fatty acid molecules.
Structure summary:
- Glycerol: a 3-carbon alcohol (C₃H₈O₃)
- Fatty acids: long hydrocarbon chains with a -COOH (carboxyl) group at one end
How It’s Made – Condensation Reaction
- Each fatty acid molecule reacts with a -OH group on the glycerol molecule.
- This reaction removes a molecule of water (H₂O) each time a bond forms.
- The new bond formed between glycerol and fatty acid is called an ester bond.
Reaction summary:
Glycerol + 3 Fatty Acids → Triglyceride + 3H₂O
🧠 Type of Bond Formed
- Ester bond (-COO-)
- Formed by condensation (water removed)
- Broken by hydrolysis (water added back)
Each ester bond = one fatty acid joined to glycerol.
🧈 (ii) Differences Between Saturated & Unsaturated Lipids
| Feature | Saturated Lipids (Fats) | Unsaturated Lipids (Oils) |
|---|---|---|
| C-C Bonds in Fatty Acids | Only single bonds (C-C) | One or more double bonds (C=C) |
| Hydrogen Content | Fully saturated with hydrogen | Not fully saturated |
| Structure | Straight chains → pack closely | Kinked chains → cannot pack tightly |
| Physical State (at room temp) | Solid (e.g., butter, animal fat) | Liquid (e.g., vegetable oil) |
| Source | Usually animal-based | Usually plant-based |
| Health Impact | Excess → may raise cholesterol | Healthier → lower cholesterol |
🧠 Mnemonic Trick
“Saturated = Solid; Unsaturated = Unsolid (liquid)”
💡 Why Lipids Are Important
- Energy storage: Provide 2× more energy per gram than carbohydrates.
- Insulation: Prevent heat loss and protect organs.
- Buoyancy: Help aquatic animals float.
- Cell membranes: Phospholipids form structural membranes.
⚡ Quick Recap
Triglyceride = Glycerol + 3 Fatty Acids
Bond formed: Ester bond (via condensation)
Reaction: 3 water molecules released
Broken by: Hydrolysis
Saturated fats: Single bonds, solid, animal fats
Unsaturated fats: Double bonds, liquid, plant oils
Easy Tip: “Saturated = Solid; Unsaturated = Oil (Liquid)”
