Edexcel A Level (IAL) Biology -2.6 Amino Acids, Proteins & Protein Structure- Study Notes- New Syllabus
Edexcel A Level (IAL) Biology -2.6 Amino Acids, Proteins & Protein Structure- Study Notes- New syllabus
Edexcel A Level (IAL) Biology -2.6 Amino Acids, Proteins & Protein Structure- Study Notes -Edexcel A level Biology – per latest Syllabus.
Key Concepts:
- 2.6 (i) know the basic structure of an amino acid Structures of specific amino acids are not required.
(ii) understand the formation of polypeptides and proteins (amino acid monomers linked by condensation reactions to form peptide bonds)
(iii) understand the significance of a protein’s primary structure in determining its secondary structure, three-dimensional structure and properties (globular and fibrous proteins and the types of bonds involved in its three-dimensional structure)
(iv) know the molecular structure of a globular protein and a fibrous protein and understand how their structures relate to their functions (including haemoglobin and collagen) - Use a semi-quantitative method to estimate protein concentration using biuret reagent and colour standards.
Understanding Amino Acids, Peptides & Protein Structure
🌱 (i) Basic Structure of an Amino Acid
Amino acids are the building blocks of proteins.
🔹 General Structure: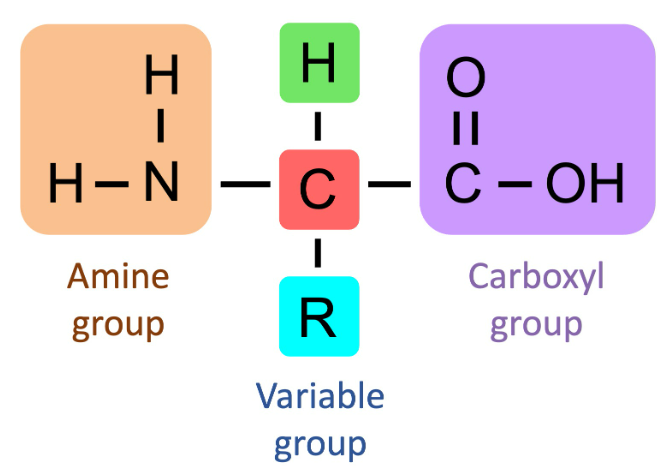
- Each amino acid has the same basic structure:
- Central carbon atom (α-carbon)
- Amino group (-NH₂)
- Carboxyl group (-COOH)
- Hydrogen atom (-H)
- R group (side chain) → differs for each amino acid
General Formula:
NH₂-CH(R)-COOH
The R group determines the type and properties of each amino acid (e.g., acidic, basic, polar, nonpolar).
Example: Glycine has R = H (simplest amino acid).
🔗 (ii) Formation of Polypeptides and Proteins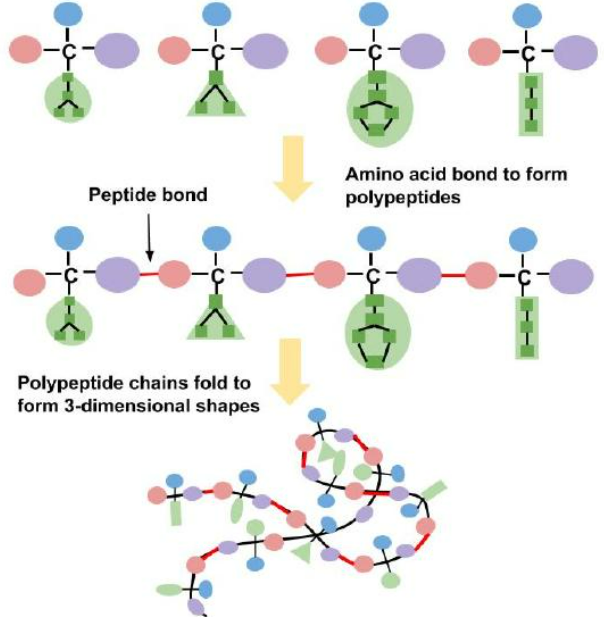
Proteins are long chains of amino acids joined together by condensation reactions.
🔹 Peptide Bond Formation:
- The carboxyl group (-COOH) of one amino acid reacts with the amino group (-NH₂) of another.
- A molecule of water (H₂O) is released.
- The resulting bond is a peptide bond (-CO-NH-).
Mnemonic: “COOH meets NH₂ → out goes H₂O → peptide bond forms.”
🔹 Chains Formed:
- 2 amino acids → dipeptide
- 3 or more → polypeptide
- Many polypeptides → protein
🧩 (iii) Protein Structure Levels & Significance
The shape (structure) of a protein determines its function. A small change in amino acid order can completely alter a protein’s function.
| Level | Description | Bonds Involved | Example / Note |
|---|---|---|---|
| Primary | Linear sequence of amino acids | Peptide bonds | Determined by DNA, a single change (e.g., in haemoglobin) can cause disease |
| Secondary | Folding of chain into α-helix or β-pleated sheet | Hydrogen bonds | Gives initial shape and strength |
| Tertiary | 3D shape (globular/fibrous) | H-bonds, ionic bonds, disulfide bridges, hydrophobic interactions | Determines overall shape & function |
| Quaternary | Multiple polypeptide chains joined | Same as tertiary + subunit interactions | e.g., haemoglobin (4 subunits) |
⚗️ (iv) Molecular Structure of Globular & Fibrous Proteins
🌐 Globular Proteins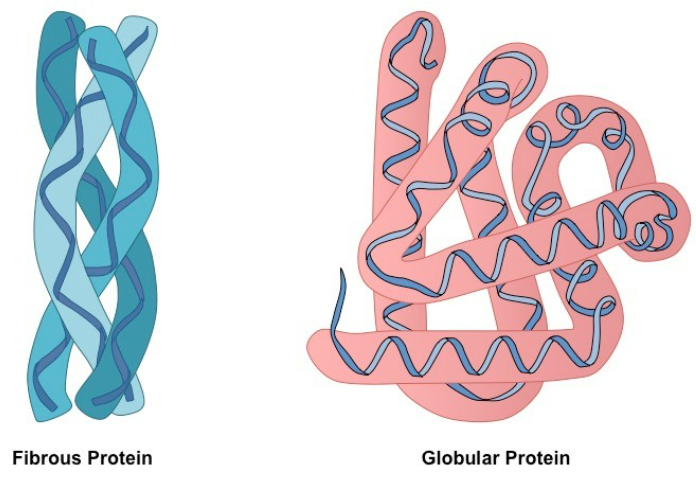
- Shape: Compact, spherical, soluble.
- Function: Metabolic (enzymes, transport, hormones).
- Bonds: Hydrogen, ionic, disulfide → maintain 3D folded shape.
- Example: Haemoglobin
- 4 polypeptide chains (2 alpha, 2 beta).
- Each contains a haem group (Fe²⁺) → binds oxygen.
- Function: Transports O₂ in blood.
- Structure-function link: Globular shape → fits through capillaries; soluble → easy transport.
🧵 Fibrous Proteins
- Shape: Long, rope-like, insoluble.
- Function: Structural (support, strength).
- Bonds: Many cross-links → tough, stable.
- Example: Collagen
- 3 polypeptide chains wound into a triple helix.
- Many hydrogen bonds between chains.
- Found in tendons, ligaments, skin.
- Structure-function link: Triple-helix + strong bonds → high tensile strength.
🧠 Summary Table
| Type | Structure | Solubility | Bonds | Function | Example |
|---|---|---|---|---|---|
| Globular | Spherical, folded | Soluble | H-bonds, ionic, disulfide | Metabolic (transport, enzyme, hormone) | Haemoglobin |
| Fibrous | Long, unbranched | Insoluble | Cross-linked, H-bonds | Structural (support, strength) | Collagen |
⚡ Quick Recap
Amino acids → building blocks of proteins
Peptide bond = CO–NH (via condensation)
Primary structure → determines higher structures
Secondary = α-helix / β-sheet (H-bonds)
Tertiary = 3D shape (H, ionic, disulfide, hydrophobic)
Quaternary = multiple chains (e.g., haemoglobin)
Globular = compact & soluble → transport/enzymes
Fibrous = long & strong → support/strength
RECOMMENDED ADDITIONAL PRACTICAL
Estimate Protein Concentration using Biuret Reagent & Colour Standards
🌱 Introduction
Proteins contain peptide bonds (-CO-NH-) that react with Biuret reagent to produce a purple/violet color.
The intensity of this color depends on how much protein is present – darker color = higher protein concentration.
This experiment uses a semi-quantitative method, meaning we compare the color of the test sample with known protein standards to estimate its concentration (not an exact numerical value, but a relative estimate).
🎯 Aim
To estimate the concentration of protein in an unknown sample using the Biuret test and color comparison with known standards.
🧰 Apparatus & Materials
- Test tubes
- Pipettes / droppers
- Test-tube rack
- Biuret reagent (alkaline copper sulfate solution)
- Protein standards (e.g., egg albumin or bovine serum albumin)
- Unknown protein solution (sample)
- Distilled water
- Color comparison chart or white background
⚗️ Reagents
Biuret Reagent Composition: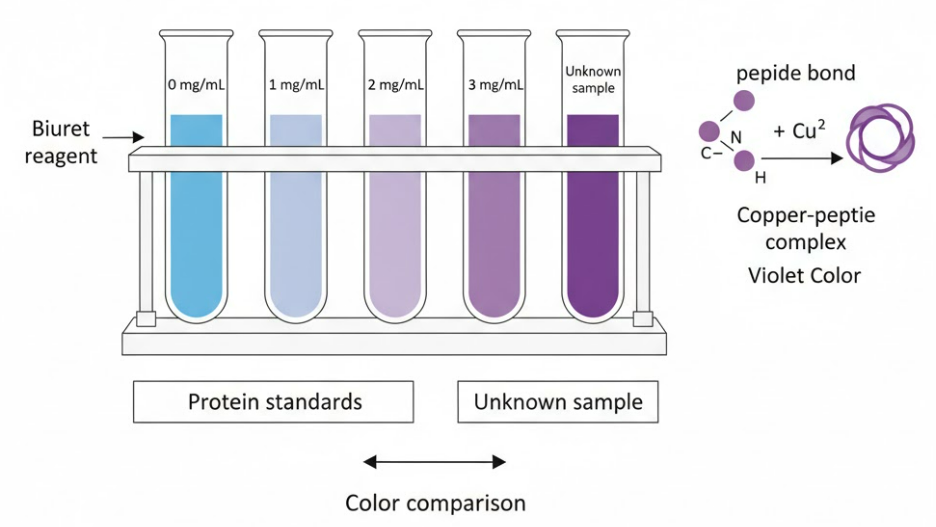
- Copper(II) sulfate (CuSO₄)
- Sodium hydroxide (NaOH)
- Potassium sodium tartrate (to stabilize Cu²⁺ ions)
🔬 Procedure
- Label test tubes for different protein concentrations, e.g., 0 mg/mL (control), 1 mg/mL, 2 mg/mL, 3 mg/mL, 4 mg/mL, and one for unknown sample.
- Pipette 2 mL of each standard solution into separate test tubes.
- Add 2 mL of Biuret reagent to each tube.
- Mix gently and leave for about 5-10 minutes at room temperature.
- Observe the color change: Light blue → violet/purple if protein present.
- Compare the color of the unknown sample with the color standards to estimate its protein concentration.
🎨 Observations
| Tube | Sample | Color After Adding Biuret | Interpretation |
|---|---|---|---|
| 1 | Distilled water (control) | Blue | No protein |
| 2 | 1 mg/mL | Light violet | Low protein |
| 3 | 2 mg/mL | Medium violet | Moderate protein |
| 4 | 3 mg/mL | Deep violet | High protein |
| 5 | Unknown sample | (Match with standard) | Estimate protein level |
🧠 Principle
Biuret reaction: peptide bonds in proteins react with Cu²⁺ ions in an alkaline medium to form a violet-colored complex.
The intensity of violet is directly proportional to the number of peptide bonds (i.e., protein concentration).
📘 Conclusion
By comparing the color intensity of the test sample with known standards, the approximate protein concentration can be estimated.
This is a semi-quantitative method – useful when exact measurement equipment (like spectrophotometers) is not available.
⚠️ Precautions
- Use equal volumes of all solutions.
- Keep time and temperature same for all tubes.
- Mix gently to avoid frothing.
- Handle Biuret reagent carefully (alkaline).
- Compare colors under same lighting conditions.
🧾 Key Notes
| Term | Meaning |
|---|---|
| Biuret test | Detects peptide bonds in proteins |
| Positive result | Violet or purple color |
| Semi-quantitative | Gives approximate, not exact, concentration |
| Color intensity | Indicates protein amount |
| Control | Distilled water → remains blue |
⚡ Quick Recap
Prepare different known protein concentrations + one unknown
Add equal volume of Biuret reagent
Wait 5–10 minutes
Observe color → light blue → violet
Compare unknown’s color with standards
💡 Darker violet = higher protein concentration
