Edexcel A Level (IAL) Biology -5.7 Separation of Photosynthetic Pigments with Chromatography- Study Notes- New Syllabus
Edexcel A Level (IAL) Biology -5.7 Separation of Photosynthetic Pigments with Chromatography- Study Notes- New syllabus
Edexcel A Level (IAL) Biology -5.7 Separation of Photosynthetic Pigments with Chromatography- Study Notes -Edexcel A level Biology – per latest Syllabus.
Key Concepts:
- 5.7 understand that chloroplast pigments can be separated using chromatography and the pigments identified using Rf values
Separation of Chloroplast Pigments Using Chromatography
🌱 Introduction
Leaf pigments like chlorophylls and carotenoids capture light for photosynthesis. Since plants contain multiple pigments, we can separate and identify them using chromatography a lab technique based on their solubility and molecular properties.
🧪 Principle of Chromatography
Chromatography separates substances based on their different affinities for the stationary phase and mobile phase.
- In paper or thin-layer chromatography (TLC):
- Stationary phase: chromatography paper or silica gel.
- Mobile phase: solvent (e.g., propanone or petroleum ether).
- Each pigment travels a different distance depending on:
- Its solubility in the solvent.
- Its interaction with the stationary phase.
🍃 Pigments Found in Chloroplasts
| Pigment | Colour | Function |
|---|---|---|
| Chlorophyll a | Blue-green | Main photosynthetic pigment (absorbs red & blue light) |
| Chlorophyll b | Yellow-green | Accessory pigment (broadens absorption range) |
| Carotene | Orange | Absorbs blue-violet light; protects chlorophyll from damage |
| Xanthophyll | Yellow | Accessory pigment; absorbs extra blue light |
⚗️ Steps for Separation
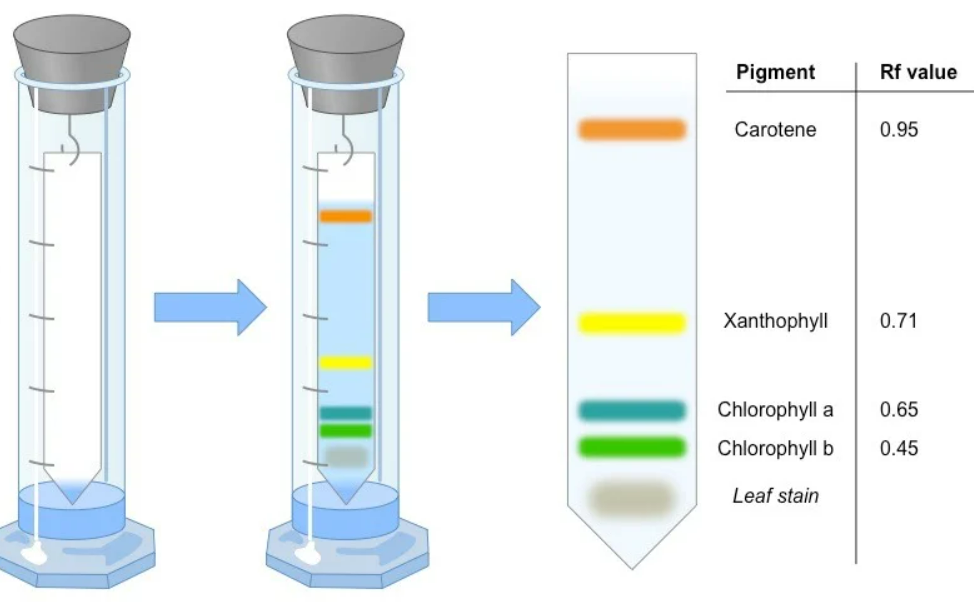
- Crush leaves in propanone to extract pigments.
- Apply a small spot of the extract onto the chromatography paper (near the base).
- Dip the paper into solvent – make sure the spot is above the solvent line.
- Allow the solvent to rise – pigments move up at different rates.
- Mark pigment bands immediately once separated.
- Measure distances for calculating Rf values.
📏 Rf Value (Retention Factor)
The Rf value helps identify pigments by comparing how far each travels relative to the solvent front.
Rf = Distance moved by pigment ÷ Distance moved by solvent front
- Rf values are always < 1.
- Different pigments → different Rf values (due to solubility & size).
- Pigments can be identified by comparing Rf values with known standards.
🎨 Typical Order of Pigments on Chromatogram (Top → Bottom)
Carotene → Xanthophyll → Chlorophyll a → Chlorophyll b
Carotene travels farthest (most soluble) – Chlorophyll b moves least (least soluble).
⚡ Quick Recap
| Concept | Key Point |
|---|---|
| Chromatography | Separates pigments by solubility & affinity |
| Main pigments | Chlorophyll a, b, carotene, xanthophyll |
| Rf value formula | Distance moved by pigment ÷ Distance moved by solvent |
| Highest Rf | Carotene |
| Lowest Rf | Chlorophyll b |
| Purpose | Identify chloroplast pigments & study light absorption roles |
💬 In short:
Chromatography separates plant pigments, and Rf values help identify them precisely revealing the colourful teamwork behind photosynthesis
