Edexcel A Level (IAL) Physics-4.32 The Nuclear Model of the Atom- Study Notes- New Syllabus
Edexcel A Level (IAL) Physics -4.32 The Nuclear Model of the Atom- Study Notes- New syllabus
Edexcel A Level (IAL) Physics -4.32 The Nuclear Model of the Atom- Study Notes -Edexcel A level Physics – per latest Syllabus.
Key Concepts:
- understand how large-angle alpha particle scattering gives evidence for a nuclear model of the atom and how our understanding of atomic structure has changed over time
Large-Angle Alpha Particle Scattering and the Nuclear Model of the Atom
Experiments involving the scattering of alpha particles provided crucial evidence that atoms have a small, dense nucleus. These results led to a major change in our understanding of atomic structure.
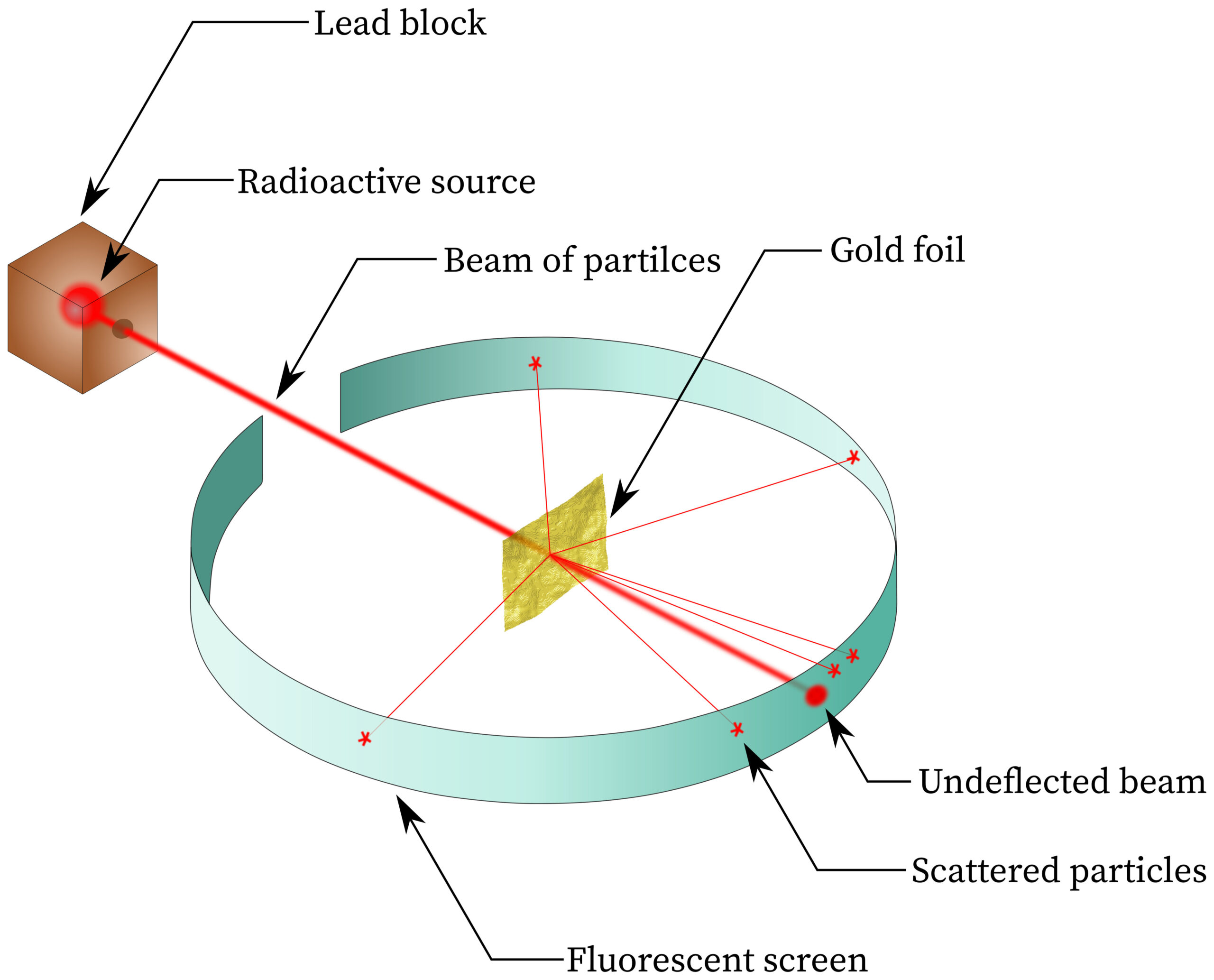
The Alpha Particle Scattering Experiment
In the experiment, a beam of alpha particles was directed at a very thin sheet of gold foil.
![]()
- Alpha particles are positively charged helium nuclei.
- They have relatively high mass and high kinetic energy.
- Their paths after passing through the foil were observed using a fluorescent screen.
Observations
- Most alpha particles passed straight through the foil with little or no deflection.
- Some alpha particles were deflected through small angles.
- A very small number were deflected through large angles (greater than \( 90^\circ \)).
- A tiny fraction were reflected almost straight back.
Why Large-Angle Scattering Was Surprising
At the time, the accepted model was the plum pudding model, where:
- Positive charge was spread uniformly throughout the atom.
- Electrons were embedded within this positive “pudding”.
This model predicted:
- Only small deflections of alpha particles.
- No large-angle scattering.
The experimental results therefore contradicted the plum pudding model.
Conclusions from the Scattering Results
- Most of the atom is empty space (explains why most alpha particles passed through).
- Positive charge and most of the mass are concentrated in a very small region.
- This central region is called the nucleus.
- The nucleus must be very dense to cause large-angle deflections.
This led to the nuclear model of the atom.
The Nuclear Model of the Atom
- A tiny, dense, positively charged nucleus at the centre.
- Electrons move around the nucleus.
- The atom is mostly empty space.
This model successfully explained large-angle alpha particle scattering.
Development of Atomic Models Over Time
(a) Dalton’s Model
- Atoms are solid, indivisible spheres.
- No internal structure.
(b) Thomson’s Plum Pudding Model
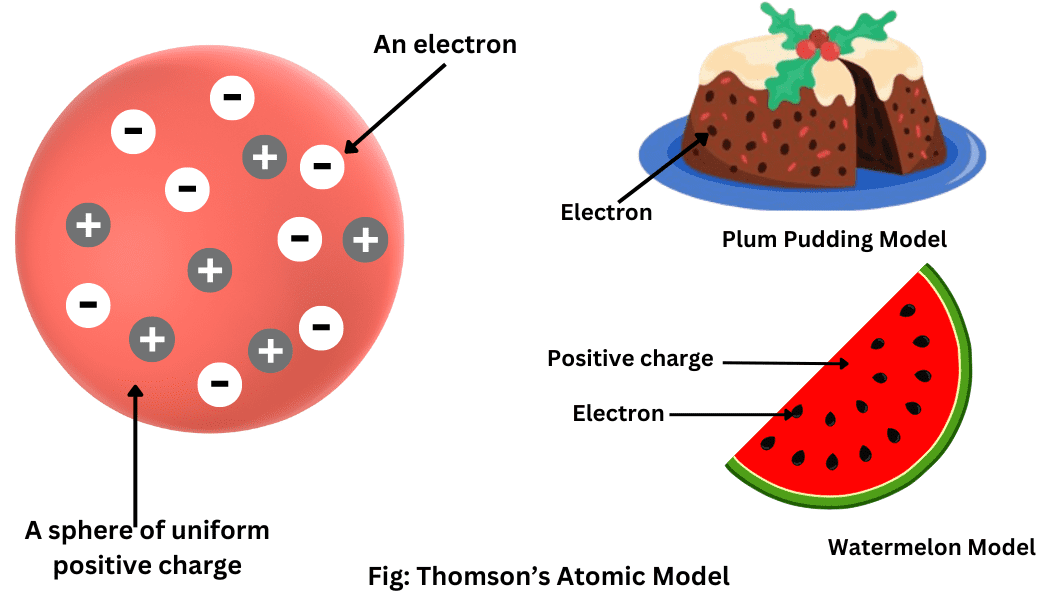
- Electrons embedded in a diffuse positive charge.
- No nucleus.
(c) Rutherford’s Nuclear Model
- Small, dense, positively charged nucleus.
- Most of atom is empty space.
(d) Bohr Model (Later Improvement)
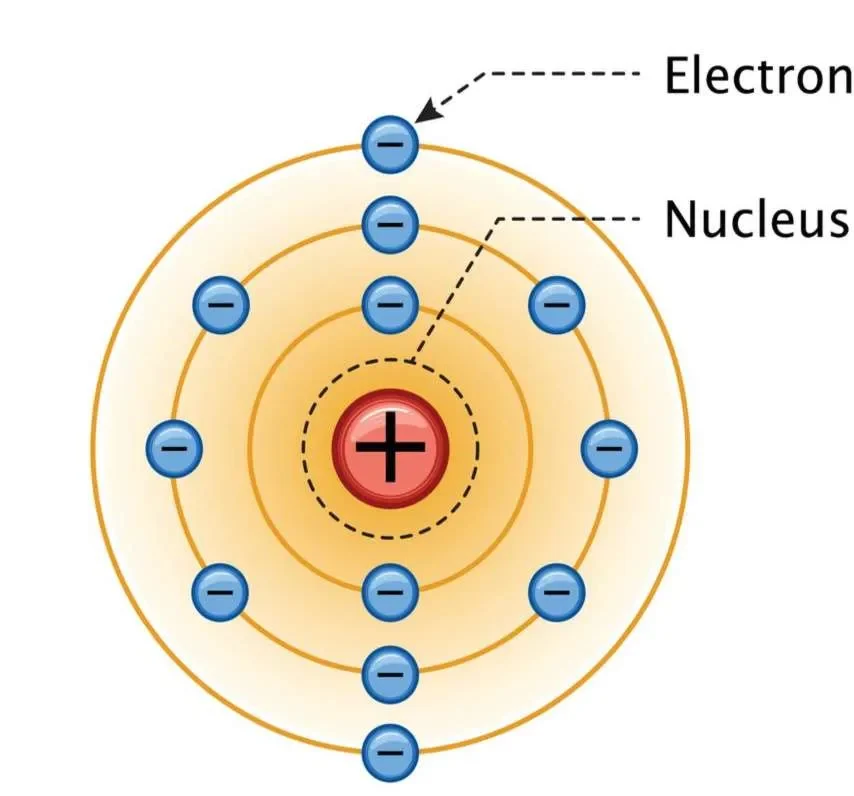
- Electrons orbit the nucleus in fixed energy levels.
- Explains atomic line spectra.
Why Large-Angle Scattering Is Key Evidence
- Only a concentrated positive nucleus can repel alpha particles so strongly.
- A spread-out positive charge cannot produce such deflections.
- Therefore, atoms must contain a compact nucleus.
Example (Easy)
State one observation from the alpha particle scattering experiment that supports the nuclear model.
▶️ Answer / Explanation
- A small number of alpha particles were deflected through large angles.
Example (Medium)
Explain why most alpha particles passed straight through the gold foil.
▶️ Answer / Explanation
- Most of the atom is empty space.
- Alpha particles rarely encounter the nucleus.
Example (Hard)
Explain why large-angle scattering could not be explained by the plum pudding model.
▶️ Answer / Explanation
- Positive charge was spread out in the plum pudding model.
- This would only cause small deflections.
- Large-angle deflections require a concentrated positive charge.
- Hence the plum pudding model fails.
