Edexcel A Level (IAL) Physics-5.11 Binding Energy per Nucleon Graph- Study Notes- New Syllabus
Edexcel A Level (IAL) Physics -5.11 Binding Energy per Nucleon Graph- Study Notes- New syllabus
Edexcel A Level (IAL) Physics -5.11 Binding Energy per Nucleon Graph- Study Notes -Edexcel A level Physics – per latest Syllabus.
Key Concepts:
- understand the processes of nuclear fusion and fission with reference to the binding energy per nucleon curve
Nuclear Fusion and Nuclear Fission Using the Binding Energy per Nucleon Curve
The processes of nuclear fusion and nuclear fission can be understood by studying the binding energy per nucleon curve, which shows how nuclear stability varies with mass number.
Binding Energy per Nucleon Curve
The binding energy per nucleon is defined as:
\( \text{binding energy per nucleon} = \dfrac{\text{total binding energy}}{\text{number of nucleons}} \)
Features of the curve:
![]()
- Binding energy per nucleon increases rapidly for light nuclei.
- The curve reaches a maximum around iron and nickel (mass number ≈ 56).
- Binding energy per nucleon decreases slowly for very heavy nuclei.
Key idea: Nuclei move toward higher binding energy per nucleon to become more stable.
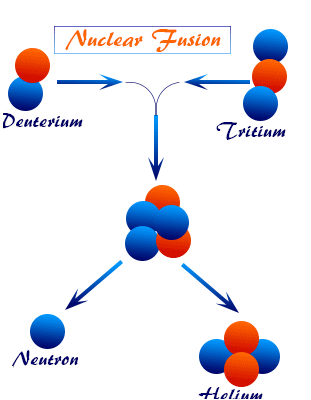
Nuclear Fusion
Nuclear fusion is the process in which two light nuclei combine to form a heavier nucleus.
- Occurs for nuclei on the left side of the binding energy curve.

- The fused nucleus has a higher binding energy per nucleon.
- The increase in binding energy corresponds to energy release.
Energy explanation:
- Total mass of product nucleus is less than the sum of the reactant masses.
- The mass difference is the mass defect.
- This mass is converted to energy using \( \Delta E = c^2 \Delta m \).
Example: Fusion of hydrogen nuclei in the Sun produces helium and releases large amounts of energy.
Why fusion releases energy:
- Products lie closer to the peak of the binding energy curve.
- Binding energy per nucleon increases.
Nuclear Fission
Nuclear fission is the process in which a heavy nucleus splits into two smaller nuclei.
- Occurs for nuclei on the right side of the binding energy curve.

- The fission products have higher binding energy per nucleon.
- The difference in binding energy is released as energy.
Energy explanation:
- Mass of fission products is less than the original nucleus.
- The mass defect is converted to energy using \( \Delta E = c^2 \Delta m \).
Example: Fission of uranium-235 in nuclear reactors releases energy used to generate electricity.
Comparison of Fusion and Fission
- Fusion: light nuclei combine → move up the curve → energy released.
- Fission: heavy nucleus splits → move down the curve toward iron → energy released.
- Both processes release energy because products are more tightly bound.
Why Iron Is the Most Stable Nucleus
- Iron lies at the peak of the binding energy per nucleon curve.
- Neither fusion nor fission of iron releases energy.
- Energy would be required to force iron nuclei to undergo nuclear reactions.
Example (Easy)
Why does nuclear fusion of light nuclei release energy?
▶️ Answer / Explanation
- Fusion products have higher binding energy per nucleon.
- They lie closer to the peak of the binding energy curve.
- The increase in binding energy is released as energy.
Example (Medium)
Why does uranium undergo fission more easily than iron?
▶️ Answer / Explanation
- Uranium lies far to the right of the binding energy curve.
- Its binding energy per nucleon is relatively low.
- Splitting moves the products closer to iron, increasing stability.
- The increase in binding energy releases energy.
Example (Hard)
Explain why both fusion and fission stop releasing energy near iron.
▶️ Answer / Explanation
- Iron has the highest binding energy per nucleon.
- Moving away from iron reduces binding energy per nucleon.
- Both fusion and fission would require energy input instead of releasing energy.