Edexcel A Level (IAL) Physics-5.15 Nuclear Decay Equations- Study Notes- New Syllabus
Edexcel A Level (IAL) Physics -5.15 Nuclear Decay Equations- Study Notes- New syllabus
Edexcel A Level (IAL) Physics -5.15 Nuclear Decay Equations- Study Notes -Edexcel A level Physics – per latest Syllabus.
Key Concepts:
- be able to write and interpret nuclear equations given the relevant particle symbols
Writing and Interpreting Nuclear Equations Using Particle Symbols
Nuclear equations describe changes in atomic nuclei during radioactive decay or nuclear reactions. They must obey conservation of nucleon number and conservation of charge.
Nuclear Notation
An атом is written as:
![]()
- \( A \) = mass (nucleon) number = protons + neutrons
- \( Z \) = proton (atomic) number
- \( X \) = chemical symbol
Relevant Particle Symbols
- Alpha particle: \( ^4_2\alpha \)
- Beta minus particle (electron): \( ^0_{-1}\beta \)
- Gamma ray: \( ^0_0\gamma \)
- Neutron: \( ^1_0 n \)
- Proton: \( ^1_1 p \)
- Antineutrino: \( \bar{\nu} \)
Note: Gamma rays carry energy but no mass or charge.
Conservation Rules
![]()
- Total mass number is conserved.
- Total charge (atomic number) is conserved.
These rules apply to all nuclear equations.
Alpha Decay
In alpha decay, the nucleus emits an alpha particle.
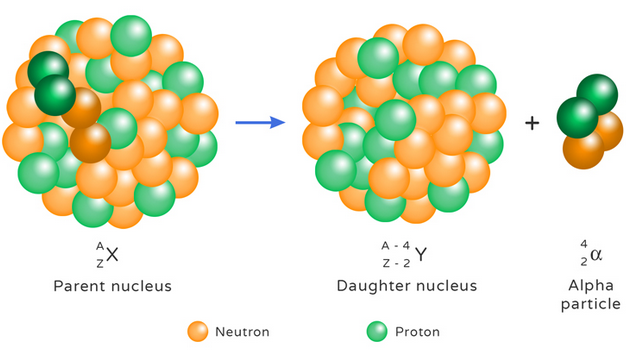
\( ^A_Z X \rightarrow ^{A-4}_{Z-2} Y + ^4_2\alpha \)
Example:
\( ^{238}_{92}\mathrm{U} \rightarrow ^{234}_{90}\mathrm{Th} + ^4_2\alpha \)
- Mass number decreases by 4
- Atomic number decreases by 2
Beta Minus Decay
In beta minus decay, a neutron changes into a proton.
\( n \rightarrow p + \beta^- + \bar{\nu} \)
At the nuclear level:
\( ^A_Z X \rightarrow ^A_{Z+1} Y + ^0_{-1}\beta + \bar{\nu} \)
![]()
Example:
\( ^{14}_{6}\mathrm{C} \rightarrow ^{14}_{7}\mathrm{N} + ^0_{-1}\beta + \bar{\nu} \)
- Mass number unchanged
- Atomic number increases by 1
Gamma Emission
Gamma emission occurs when a nucleus releases excess energy.
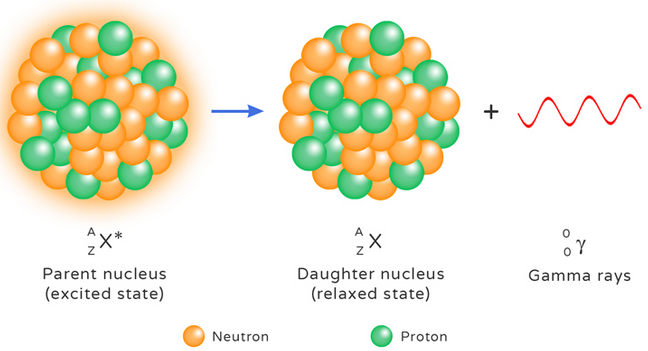
\( ^A_Z X^* \rightarrow ^A_Z X + ^0_0\gamma \)
- No change in mass number
- No change in atomic number
Interpreting Nuclear Equations
To interpret a nuclear equation:
- Identify the emitted particle.
- Check conservation of mass number.
- Check conservation of charge.
- Determine the change in the nucleus.
Example (Easy)
Identify the type of decay:
\( ^{226}_{88}\mathrm{Ra} \rightarrow ^{222}_{86}\mathrm{Rn} + ^4_2\alpha \)
▶️ Answer / Explanation
This is alpha decay because an alpha particle is emitted.
Example (Medium)
Complete the equation:
\( ^{90}_{38}\mathrm{Sr} \rightarrow \; ? \; + ^0_{-1}\beta \)
▶️ Answer / Explanation
Atomic number increases by 1, mass number unchanged:
\( ^{90}_{38}\mathrm{Sr} \rightarrow ^{90}_{39}\mathrm{Y} + ^0_{-1}\beta \)
Example (Hard)
Explain why gamma emission is often written after alpha or beta decay.
▶️ Answer / Explanation
- Alpha or beta decay may leave the nucleus in an excited state.
- Gamma emission removes excess energy.
- The identity of the nucleus remains unchanged.
