Edexcel A Level (IAL) Physics-5.18 Equations for Nuclear Physics- Study Notes- New Syllabus
Edexcel A Level (IAL) Physics -5.18 Equations for Nuclear Physics- Study Notes- New syllabus
Edexcel A Level (IAL) Physics -5.18 Equations for Nuclear Physics- Study Notes -Edexcel A level Physics – per latest Syllabus.
Key Concepts:
- Be able to determine the half-lives of radioactive isotopes graphically and be able to use the equations for radioactive decay activity \(A = \lambda N\), \(\dfrac{dN}{dt} = -\lambda N\), \(\lambda = \dfrac{\ln 2}{t_{1/2}}\), \(N = N_0 e^{-\lambda t}\) and \(A = A_0 e^{-\lambda t}\) and derive and use the corresponding log equations.
Half-Life, Radioactive Decay Equations and Logarithmic Forms
Radioactive decay describes how the number of unstable nuclei and the activity of a radioactive source decrease with time. This behaviour can be analysed graphically and using exponential and logarithmic equations.
Activity and Number of Nuclei
Activity is the rate at which nuclei decay.![]()
\( A = \lambda N \)
- \( A \) = activity (Bq)
- \( N \) = number of undecayed nuclei
- \( \lambda \) = decay constant (s⁻¹)
Meaning: Higher number of nuclei → higher activity.
Radioactive Decay Law
The rate of decay of nuclei is proportional to the number remaining:
\( \dfrac{dN}{dt} = -\lambda N \)
- Negative sign indicates that \( N \) decreases with time.
- The decay constant \( \lambda \) is a property of the isotope.
Exponential Decay Equation
Solving the decay equation gives:![]()
\( N = N_0 e^{-\lambda t} \)
- \( N_0 \) = initial number of nuclei
- \( t \) = time
Since activity is proportional to \( N \):
\( A = A_0 e^{-\lambda t} \)
Half-Life and Decay Constant
Half-life \( t_{1/2} \) is the time taken for half the nuclei to decay.
The relationship between half-life and decay constant is:
\( \lambda = \dfrac{\ln 2}{t_{1/2}} \)![]()
Important: Half-life is constant for a given isotope.
Determining Half-Life Graphically
Method:
- Plot activity (or count rate) against time.
- Subtract background radiation first.
- Choose any value of activity.
- Find the time taken for activity to fall to half that value.
- This time interval is the half-life.
Note: The half-life is the same anywhere on the decay curve.
Logarithmic Form of the Decay Equation
Starting from:
\( N = N_0 e^{-\lambda t} \)
Take natural logarithms:
\( \ln N = \ln N_0 – \lambda t \)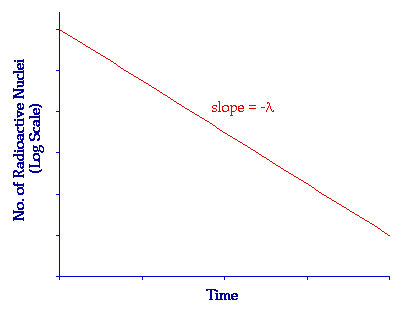
Similarly for activity:
\( \ln A = \ln A_0 – \lambda t \)
Using Log Graphs
- Plot \( \ln A \) against \( t \).
- The graph is a straight line.
- Gradient = \( -\lambda \).
- Intercept = \( \ln A_0 \).
Half-life can then be found using:
\( t_{1/2} = \dfrac{\ln 2}{\lambda} \)
Example (Easy)
A radioactive source has an activity of 800 Bq. Find the activity after one half-life.
▶️ Answer / Explanation
After one half-life:
\( A = \dfrac{800}{2} = 400\ \mathrm{Bq} \)
Example (Medium)
The half-life of a substance is \( 10\,\mathrm{days} \). Find the decay constant.
▶️ Answer / Explanation
\( \lambda = \dfrac{\ln 2}{10} = 0.0693\ \mathrm{day^{-1}} \)
Example (Hard)
A radioactive source has initial activity \( 5000\ \mathrm{Bq} \). After 30 s, the activity is 1250 Bq. Calculate the half-life.
▶️ Answer / Explanation
Activity has fallen by a factor of 4 → two half-lives.
\( 30\ \mathrm{s} = 2 t_{1/2} \)
\( t_{1/2} = 15\ \mathrm{s} \)
