Edexcel A Level (IAL) Physics-5.3 Core Practical 13: Investigating Specific Latent Heat- Study Notes- New Syllabus
Edexcel A Level (IAL) Physics -5.3 Core Practical 13: Investigating Specific Latent Heat- Study Notes- New syllabus
Edexcel A Level (IAL) Physics -5.3 Core Practical 13: Investigating Specific Latent Heat- Study Notes -Edexcel A level Physics – per latest Syllabus.
Key Concepts:
- CORE PRACTICAL 13: Determine the specific latent heat of a phase change
CORE PRACTICAL 13: Determine the Specific Latent Heat of a Phase Change
This experiment is used to determine the specific latent heat of a substance during a change of state, such as melting or boiling, without a change in temperature.
Aim
To determine the specific latent heat of fusion or vaporisation of a substance using electrical heating.
Key Theory
When a substance changes phase:
- Temperature remains constant
- Energy is transferred to break or form intermolecular bonds
The relationship between energy and mass is:
\( E = mL \)
- \( E \) = energy transferred (J)
- \( m \) = mass undergoing phase change (kg)
- \( L \) = specific latent heat (J kg⁻¹)
Electrical energy supplied is calculated using:
\( E = Pt = VIt \)
Apparatus
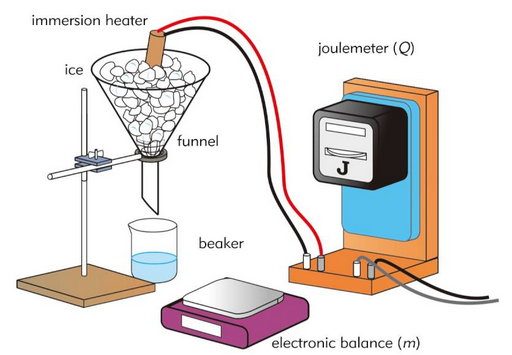
- Power supply
- Immersion heater
- Ammeter
- Voltmeter
- Stopwatch
- Electronic balance
- Insulated container
- Substance (e.g. ice or water)
Method
- Measure the initial mass of the substance.
- Insert the immersion heater fully into the substance.
- Switch on the power supply and start the stopwatch.
- Record the voltage \( V \) and current \( I \).
- Allow the substance to undergo a phase change at constant temperature.
- After a known time \( t \), switch off the heater.
- Measure the final mass.
- Calculate the mass change \( m \).
Calculation
- Energy supplied: \( E = VIt \)
- Specific latent heat: \( L = \dfrac{E}{m} \)
Assumptions
- All electrical energy causes the phase change
- Heat losses to surroundings are small
- Temperature remains constant during phase change
Sources of Error
- Heat loss to surroundings
- Incomplete immersion of heater
- Evaporation losses (for boiling experiments)
- Inaccurate mass measurement
Improvements
- Use better insulation
- Repeat readings and average
- Use a lid to reduce heat loss
- Use a data logger for current and voltage
Example
An immersion heater operates at \( 12.0\,\mathrm{V} \) and \( 3.0\,\mathrm{A} \) for \( 300\,\mathrm{s} \). The mass of ice melted is \( 0.010\,\mathrm{kg} \). Calculate the specific latent heat of fusion.
▶️ Answer / Explanation
Energy supplied:
\( E = VIt = 12.0 \times 3.0 \times 300 = 10800\,\mathrm{J} \)
Specific latent heat:
\( L = \dfrac{10800}{0.010} = 1.08\times10^{6}\,\mathrm{J\,kg^{-1}} \)
Specific latent heat = \( 1.08\times10^{6}\,\mathrm{J\,kg^{-1}} \)
