IB DP Biology A1.1 Water Exam Style Questions HL Paper 1
IB DP Biology A1.1 Water Exam Style Questions HL Paper 1
IB DP Biology A1.1 Water Exam Style Questions HL Paper 1 at IITian Academy focus on a specific topic or type of questions asked in actual exam. Questions are based on new syllabus for first assessment of 2025.
A1.1 Water Topics : Polarity, Hydrogen Bonding, Solvent Properties, Physical Properties, Consequences, Extraterrestrial Life
Question
Which symbol indicates the polarity of the oxygen atom in the water molecule?
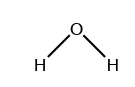
(B) δ⁻
(C) +
(D) −
▶️ Answer/Explanation
✅ Answer: (B)
Question
Which property of water helps aquatic habitats maintain stable temperatures throughout the year?
A. High specific heat capacity because covalent bonding restricts molecular motion
B. Low specific heat capacity because covalent bonding restricts molecular motion
C. Low specific heat capacity because hydrogen bonding restricts molecular motion
D. High specific heat capacity because hydrogen bonding restricts molecular motion
▶️ Answer/Explanation
✅ Answer: (D) High specific heat capacity because hydrogen bonding restricts molecular motion
Question
The mean air and water temperatures recorded at the same time of day at various distances downstream from the city of Asunción on the Lower Paraguay River over an 8-day period shows a relation. What accounts for the differences between the water and air temperatures?
A. Evaporation of surface water causes an increase in the temperature of surface water.
B. Adhesion between water molecules prevents heat absorption, so its temperature remains lower.
C. Heat is rapidly lost from water due to breakage of covalent bonds.
D. Breakage of hydrogen bonds in water requires much heat energy.
▶️Answer/Explanation
Answer: D. Breakage of hydrogen bonds in water requires much heat energy.
Explanation:
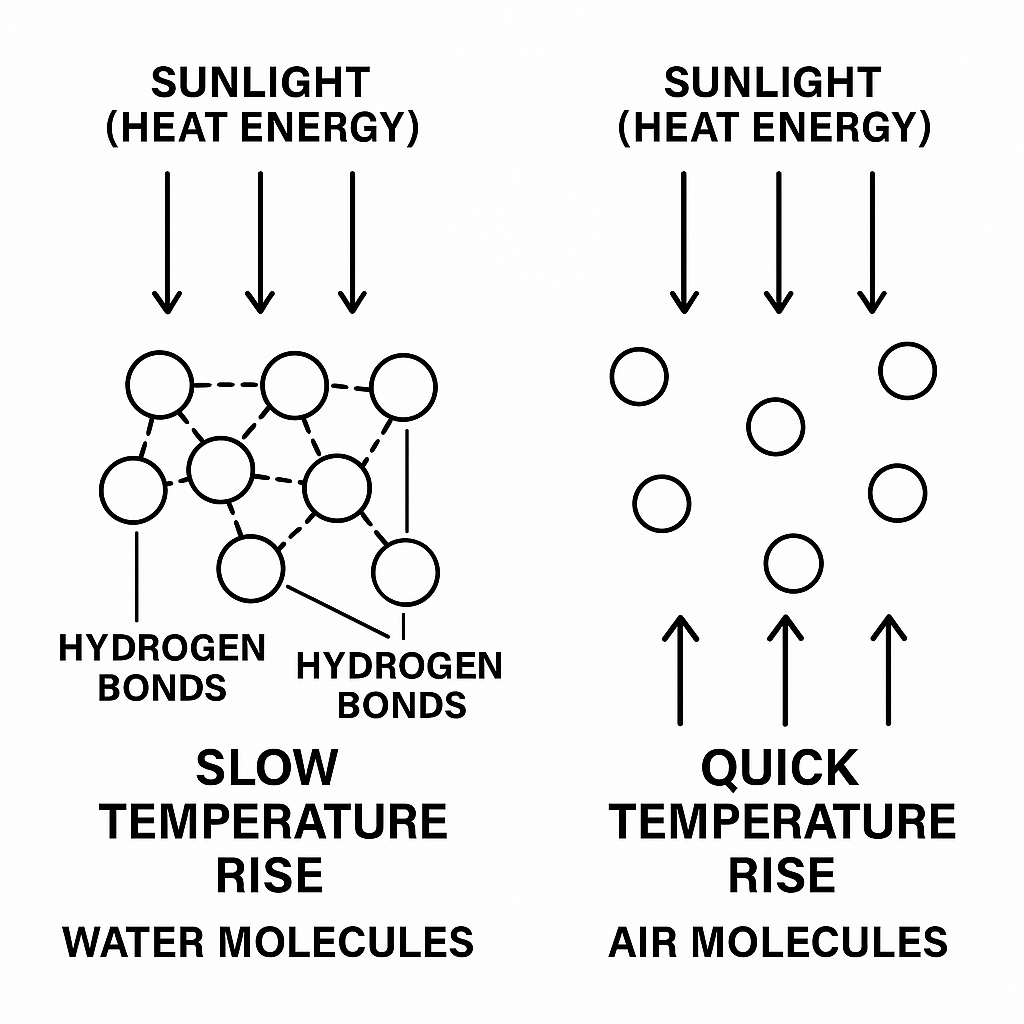 Water has a special property due to hydrogen bonding between its molecules. These bonds are weak, but there’s a lot of them, and they require a good amount of heat energy to break. So, when heat is applied to water (like from the Sun), a large part of that energy goes into breaking these hydrogen bonds instead of raising the temperature right away.
Water has a special property due to hydrogen bonding between its molecules. These bonds are weak, but there’s a lot of them, and they require a good amount of heat energy to break. So, when heat is applied to water (like from the Sun), a large part of that energy goes into breaking these hydrogen bonds instead of raising the temperature right away.
This is why water heats up more slowly than air. Even though both are exposed to the same sunlight, the air gets hotter faster, while the water stays cooler for longer. This explains why water and air can have different temperatures even at the same location and time.
