IB DP Biology- C1.1 Enzymes and metabolism - IB Style Questions For SL Paper 1A -FA 2025
Question
The diagram demonstrates how an enzyme–substrate complex is formed, showing a substrate approaching the enzyme’s active site and the resulting complex.
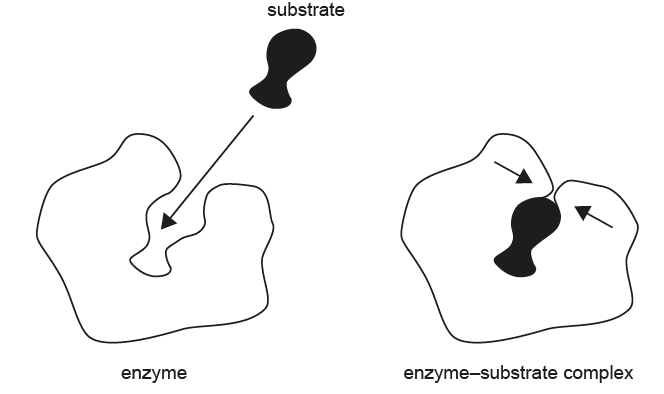

Which statement best explains the process illustrated in the diagram?
(A) The lock-and-key hypothesis, where the substrate fits the enzyme perfectly
(B) The substrate permanently changes the shape of the enzyme’s active site
(C) The substrate and the active site possess identical shapes
(D) The active site adjusts its shape to fit the substrate, as described by the induced-fit model
(B) The substrate permanently changes the shape of the enzyme’s active site
(C) The substrate and the active site possess identical shapes
(D) The active site adjusts its shape to fit the substrate, as described by the induced-fit model
▶️ Answer/Explanation
Detailed solution
In the diagram, the enzyme’s active site shifts shape when the substrate binds. This behavior is characteristic of the induced-fit model, where the enzyme molds itself slightly around the substrate to ensure a precise fit. The active site is not rigid, nor does the substrate permanently modify its shape.
✅ Answer: (D)
Question
What is the advantage of using lactase in an immobilized state in the food manufacturing industry?
A. It functions within cells.
B. It dissolves in multiple solvents.
C. It converts cellulose into glucose.
D. It is less likely to become denatured.
▶️ Answer/Explanation
Answer: D. It is less likely to become denatured.
Explanation:
Immobilizing lactase (attaching the enzyme to a solid support such as beads or a matrix) increases its structural stability. This reduces the likelihood of denaturation during processing (e.g., shifts in temperature or pH), allows the enzyme to be reused, and facilitates separation from the product stream—improving efficiency and lowering costs in food manufacturing (e.g., producing lactose-reduced milk).
Options Evaluation:
A. Incorrect — Industrial lactase is typically used outside cells and is often immobilized to enhance stability and reuse.
B. Incorrect — Enzymes, including lactase, are proteins that function best in aqueous environments; they are not designed to dissolve or act in multiple solvents.
C. Incorrect — Lactase catalyzes the hydrolysis of lactose into glucose and galactose. Cellulose is broken down by cellulase, not lactase.
D. Correct — Immobilization makes the enzyme more resistant to denaturation and suitable for repeated use.
Explanation:
Immobilizing lactase (attaching the enzyme to a solid support such as beads or a matrix) increases its structural stability. This reduces the likelihood of denaturation during processing (e.g., shifts in temperature or pH), allows the enzyme to be reused, and facilitates separation from the product stream—improving efficiency and lowering costs in food manufacturing (e.g., producing lactose-reduced milk).
Options Evaluation:
A. Incorrect — Industrial lactase is typically used outside cells and is often immobilized to enhance stability and reuse.
B. Incorrect — Enzymes, including lactase, are proteins that function best in aqueous environments; they are not designed to dissolve or act in multiple solvents.
C. Incorrect — Lactase catalyzes the hydrolysis of lactose into glucose and galactose. Cellulose is broken down by cellulase, not lactase.
D. Correct — Immobilization makes the enzyme more resistant to denaturation and suitable for repeated use.
