IB DP Biology- D2.3 Water potential - IB Style Questions For SL Paper 1A -FA 2025
Question
The diagram below shows human red blood cells placed in a particular solution.
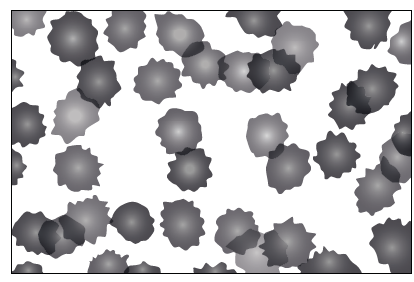
Based on the appearance of the cells, what can be concluded about the type of solution they are in?
A. The solution is hypotonic as the cells are crenated.
B. The solution is hypotonic as the cells are turgid.
C. The solution is hypertonic as the cells are crenated.
D. The solution is hypertonic as the cells are turgid.
▶️ Answer/Explanation
This occurs when the surrounding solution has a higher solute concentration — a hypertonic environment — causing the cells to lose water and become crenated.
✅ Answer: (C) The solution is hypertonic as the cells are crenated.
Question
What feature do diffusion and osmosis share?
A. They only occur inside living cells.
B. They require membrane transport proteins.
C. They are passive transport mechanisms.
D. Net movement of substances goes against the concentration gradient.
▶️ Answer/Explanation
Correct answer: (C) They are passive transport mechanisms.
Both diffusion and osmosis involve movement of particles from an area of higher concentration to an area of lower concentration without the need for cellular energy, making them passive processes.
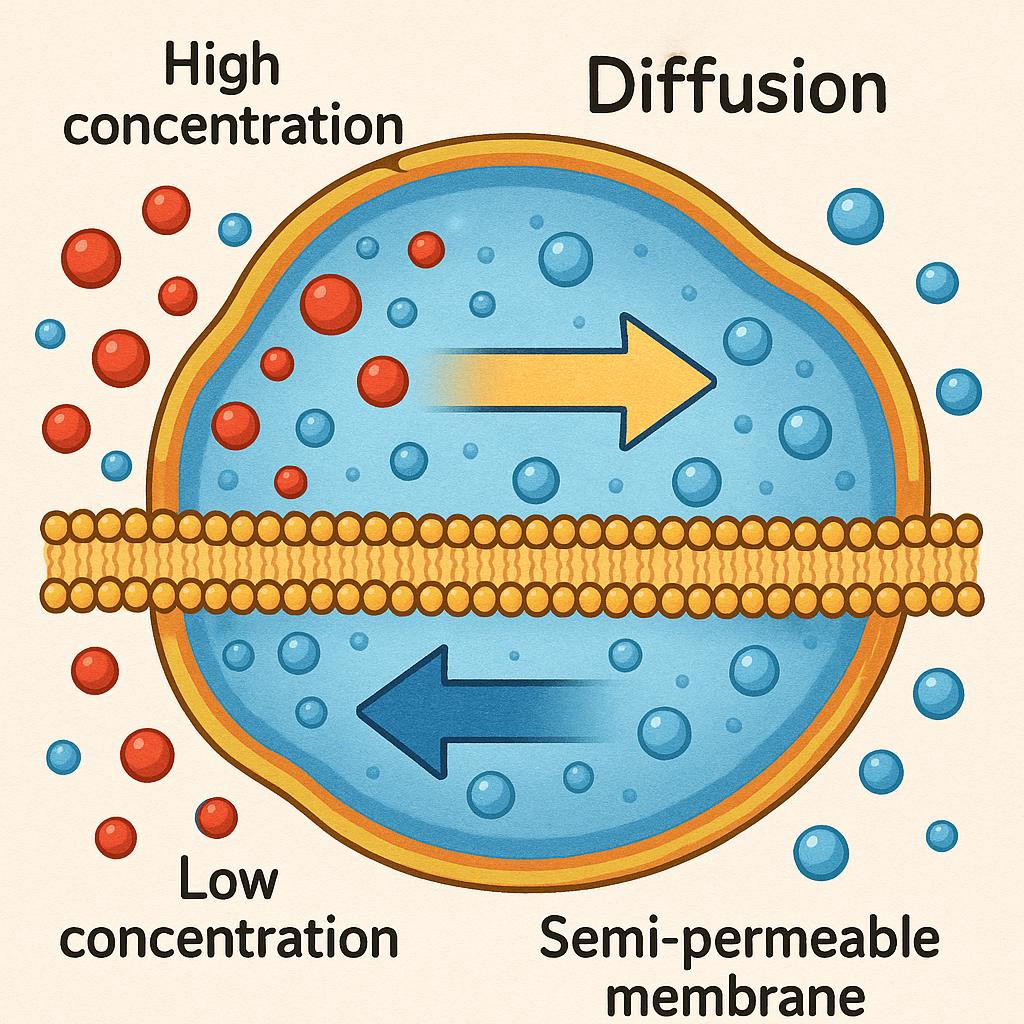 Diffusion is the movement of solute molecules, while osmosis refers specifically to the movement of water across a selectively permeable membrane.
Diffusion is the movement of solute molecules, while osmosis refers specifically to the movement of water across a selectively permeable membrane.
Why other choices are incorrect:
A. Diffusion and osmosis occur in both living and non-living systems.
B. Transport proteins are needed for facilitated diffusion or active transport, not simple diffusion or osmosis.
D. Both processes move substances down the concentration gradient, not against it.
✅ Answer: (C)
Question
Which process(es) occur(s) by osmosis?
I. Uptake of water by cells in the wall of the intestine
II. Loss of water from a plant cell in a hypertonic environment
III. Evaporation of water from sweat on the skin surface
A. I only
B. I and II only
C. II and III only
D. I, II and III
▶️ Answer/Explanation
Osmosis is the passive movement of water across a selectively permeable membrane from higher water potential (lower solute concentration) to lower water potential (higher solute concentration).
Analysis of statements:
I. Correct – Water enters intestinal wall cells by osmosis because solute concentration is higher inside the cells than in the gut lumen.
II. Correct – In a hypertonic environment, water moves out of a plant cell by osmosis, leading to plasmolysis.
III. Incorrect – Evaporation is a physical change from liquid water to vapor; it does not involve membrane transport, so it is not osmosis.
✅ Answer: (B)
