IB Biology SL (Standard level)- 2024 – Practice Questions- All Topics
Topic 2.2 Water
Topic 2 Weightage : 20%
All Questions for Topic 2.2- Water Structure, Hydrogen Bonding, Thermal Properties,Cohesive and Adhesive Properties,Solvent Properties, Significance of Water, Water Cycle
Question
What is the term for the attraction of water molecules to other water molecules?
Surface tension
Capillary action
Cohesion
Adhesion
▶️Answer/Explanation
Ans: C
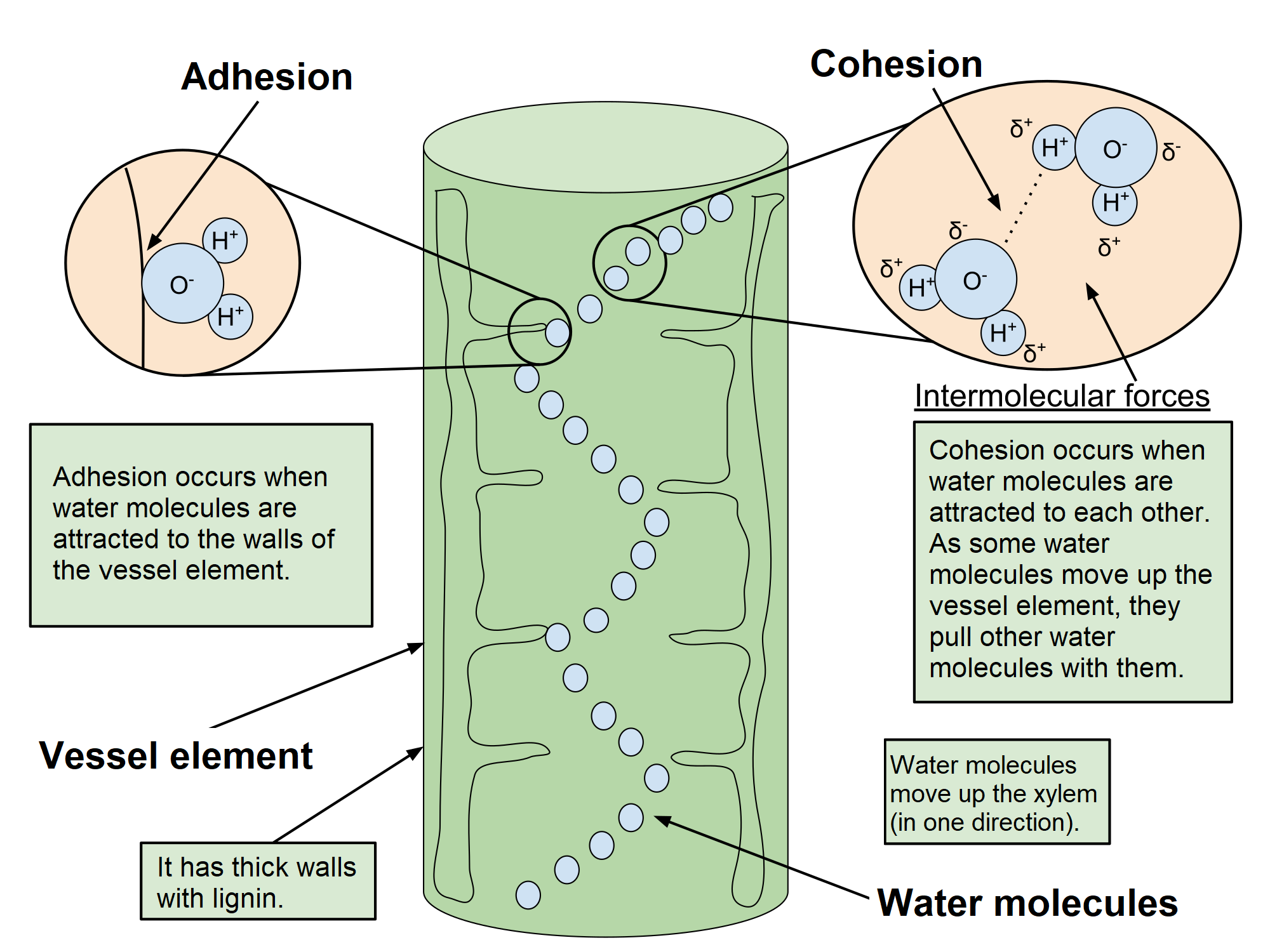
Cohesion is the attraction of water molecules to other water molecules due to hydrogen bonding. Each water molecule can form four hydrogen bonds with neighbor molecules. The strong Coulomb attraction between the molecules draws them together or makes them “sticky”. Cohesion is important in plants because it helps them take up water at their roots. Cohesion also contributes to water’s high boiling point, which helps animals regulate body temperature.
Question
Between which atoms do hydrogen bonds form in water?
A. Oxygen and hydrogen atoms in the same water molecule
B. Oxygen and hydrogen atoms in different water molecules
C. Hydrogen atoms in the same water molecule
D. Oxygen atoms of different water molecules
▶️Answer/Explanation
Markscheme
Ans: B
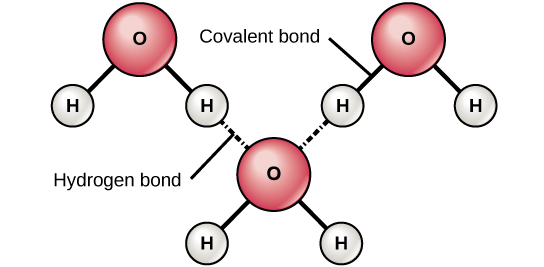
Hydrogen bonds form between the hydrogen atoms of one water molecule and the oxygen atom of another water molecule. Each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in the molecule.
Question
Which of these molecules is a disaccharide?
A. Galactose
B. Sucrose
C. Cellulose
D. Ribose
▶️Answer/Explanation
Markscheme
B
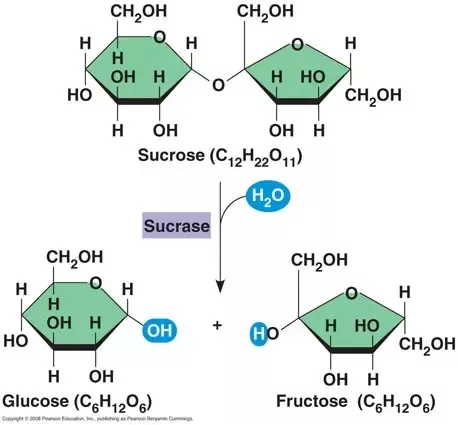
Sucrose is a disaccharide because it is composed of two monosaccharides, glucose and fructose, that are joined by a glycosidic bond. Hydrolysis of sucrose by the enzyme invertase yields “invert sugar,” a 50:50 mixture of fructose and glucose.
