IB Biology HL (HIGHER level)- 2024 – Practice Questions- All Topics
Topic 8.1 Metabolism
Topic 8 Weightage : 13%
All Questions for Topic 8.1 – Metabolic Pathways, Activation Energy, Enzyme Inhibition, Feedback Inhibition, Enzyme Kinetics, Rational Drug Design, Models of Action, Allosterism, Yeast-2-Hybrid System
Question
Succinate dehydrogenase is an enzyme that catalyses the oxidation of succinic acid. If malonic acid is added to the mixture, the rate of reaction is reduced. An increase in succinic acid will increase the rate of reaction again. For this system, which term best describes malonic acid?
A Substrate
B End product
C Non-competitive inhibitor
D Competitive inhibitor
▶️Answer/Explanation
Ans: D
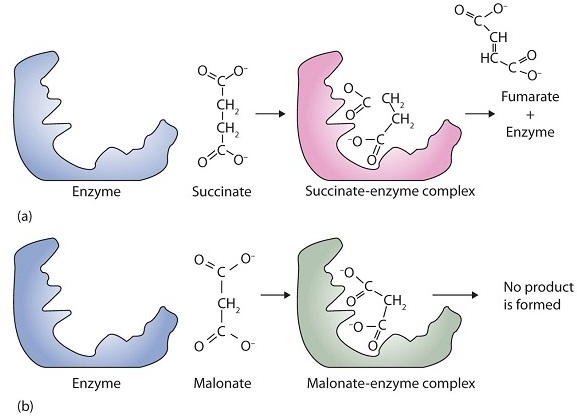
Malonic acid is a competitive inhibitor. A competitive inhibitor is a type of enzyme inhibitor that competes with the substrate for binding to the active site of the enzyme. In this case, malonic acid competes with succinic acid for binding to the active site of succinate dehydrogenase, which reduces the rate of the reaction. When the concentration of succinic acid is increased, it outcompetes malonic acid and binds to the active site of the enzyme, increasing the rate of the reaction.
Question
Which statement applies to the tertiary structure of enzymes?
A Tertiary structure is the sequence of amino acids in an enzyme.
B Some enzymes do not have a tertiary structure.
C An example of tertiary structure in an enzyme is the alpha helix.
D A change in the tertiary structure of an enzyme may result in a change in the structure of the active site.
▶️Answer/Explanation
Ans: D
D. A change in the tertiary structure of an enzyme may result in a change in the structure of the active site because the tertiary structure of an enzyme is the three-dimensional shape that the protein folds into. This shape is determined by the interactions between the amino acid side chains that make up the protein. The active site of an enzyme is a specific region of the protein that binds to the substrate and catalyzes the reaction. A change in the tertiary structure of the enzyme can alter the shape of the active site, which can affect the enzyme’s ability to bind to the substrate and catalyze the reaction. This is why a change in the tertiary structure of an enzyme may result in a change in the structure of the active site.
The graph shows an example of an enzyme-catalysed reaction.
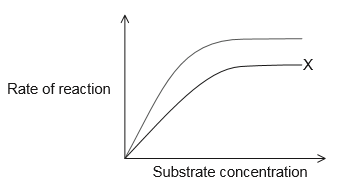
What does the curve labelled X represent?
A. No inhibition
B. Competitive inhibition
C. Non-competitive inhibition
D. Reversible inhibition
▶️Answer/Explanation
Markscheme
C
With a noncompetitive inhibitor, the reaction can never reach its normal $V_{max}$, regardless of how much substrate we add. A subset of the enzyme molecules will always be “poisoned” by the inhibitor, so the effective concentration of enzyme (which determines $V_{max}$ ) is reduced. However, the reaction reaches half of its new $V_{max}$ at the same substrate concentration, so $K_{m}$ is unchanged. The unchanged $K_{m}$ reflects that the inhibitor doesn’t affect binding of enzyme to substrate, just lowers the concentration of usable enzyme. Thus, the curve labelled X represent non-competitive inhibition.
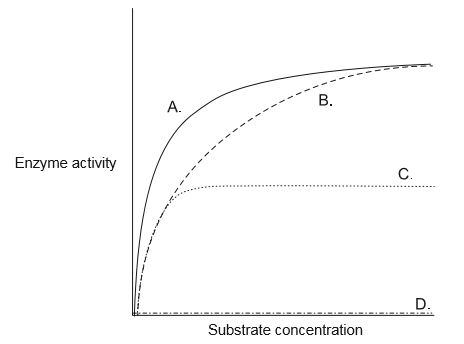
In one of the curves in the graph, the rate of an enzyme-catalysed reaction has been plotted against the substrate concentration in presence of a small quantity of a competitive inhibitor. Which curve represents competitive inhibition?
▶️Answer/Explanation
Markscheme
B
Competitive inhibitors impair reaction progress by binding to an enzyme, often at the active site, and preventing the real substrate from binding. At any given time, only the competitive inhibitor or the substrate can be bound to the enzyme (not both). That is, the inhibitor and substrate compete for the enzyme. Competitive inhibition acts by decreasing the number of enzyme molecules available to bind the substrate.
With a competitive inhibitor, the reaction can eventually reach its normal $V_{max}$, but it takes a higher concentration of substrate to get it there. In other words, $V_{max}$ is unchanged, but the apparent $K_{m}$ is higher. The extra substrate makes the substrate molecules abundant enough to consistently “beat” the inhibitor molecules to the enzyme.
Which describes the role of amino acids in the channels of membrane proteins used for facilitated diffusion?
A. Polar amino acids create a channel through which hydrophilic molecules can pass.
B. Polar amino acids create a channel through which hydrophobic molecules can pass.
C. Non-polar amino acids create a channel through which hydrophilic molecules can pass.
D. Non-polar amino acids create a channel through which hydrophobic molecules can pass.
▶️Answer/Explanation
Markscheme
A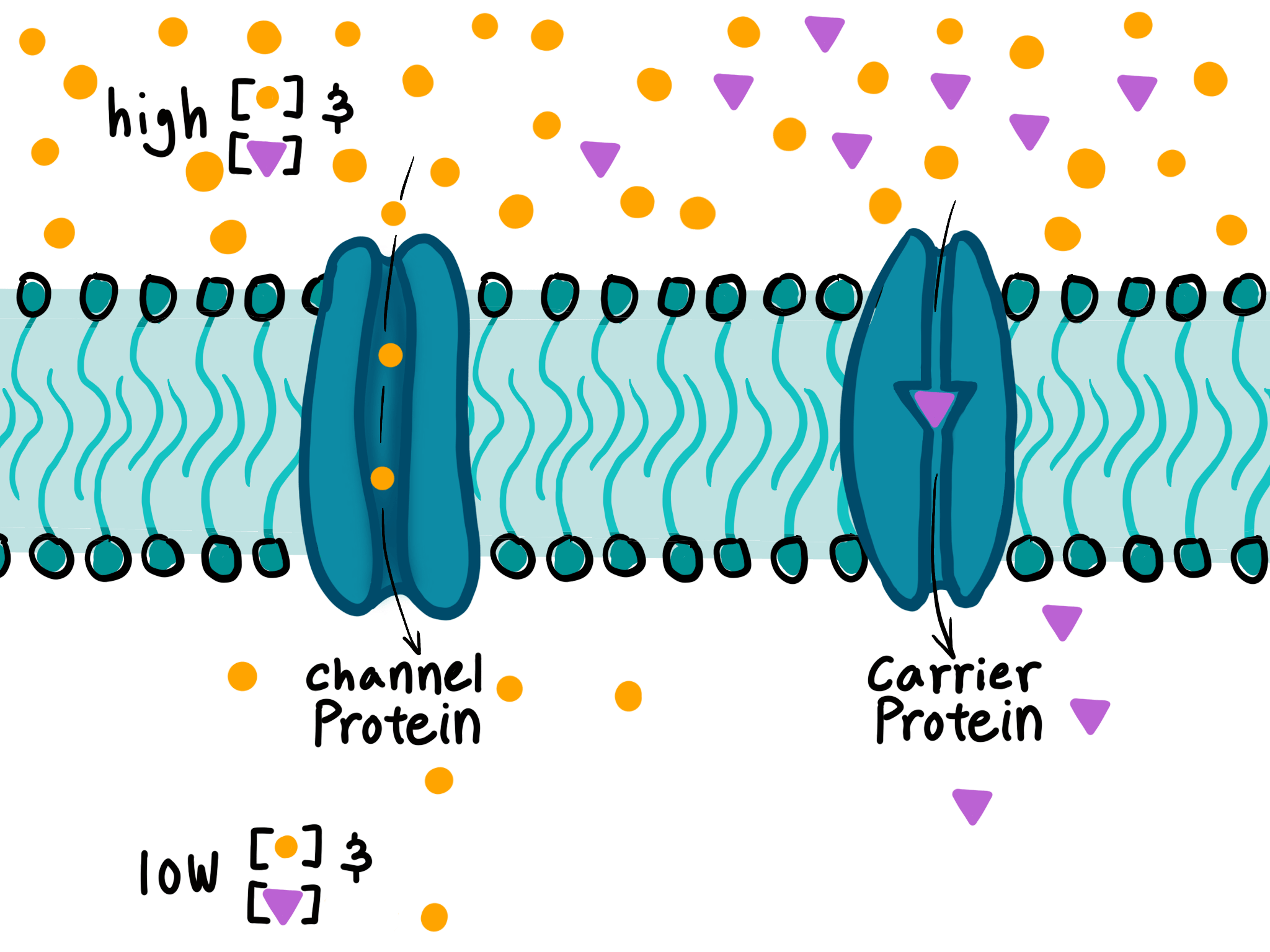
Option A is correct because channel proteins used for facilitated diffusion are composed of polar amino acids that create a hydrophilic channel. Hydrophilic molecules can pass through this channel because they are attracted to the charged amino acids that line the channel. Non-polar amino acids, which are hydrophobic, do not create a hydrophilic channel and are not involved in the transport of hydrophilic molecules. Therefore, option A is the most accurate description of the role of amino acids in the channels of membrane proteins used for facilitated diffusion.
Question
Antifreeze is a compound called ethylene glycol, which is metabolized in mammals to poisonous compounds that cause kidney failure, amongst other symptoms. The first step in metabolism involves an enzyme called alcohol dehydrogenase. Two inhibitors of this enzyme used in treating antifreeze poisoning are ethanol and fomepizole. The chemical structures of antifreeze and the two inhibitors are shown.
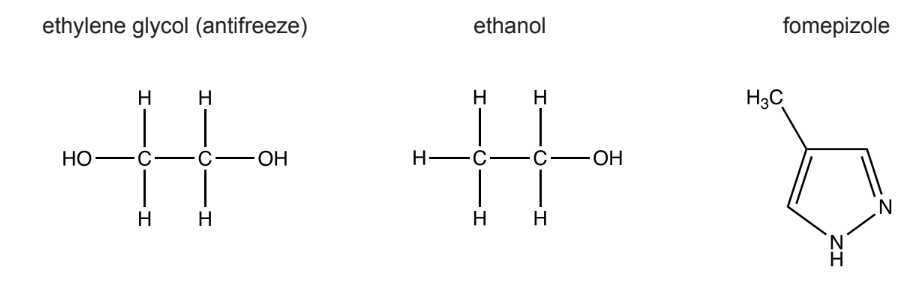
Studies have shown that fomepizole may be preferred to ethanol as a treatment. What is a reason for this?
A. Fomepizole is a competitive inhibitor and may be given in small doses.
B. Ethanol is a non-competitive inhibitor and may be given in small doses.
C. Fomepizole is a non-competitive inhibitor and must be given in very large doses.
D. Ethanol is a competitive inhibitor and must be given in very large doses.
▶️Answer/Explanation
Ans:D
Ethanol is a competitive inhibitor of alcohol dehydrogenase, which is the enzyme responsible for the metabolism of ethylene glycol. Ethanol competes with ethylene glycol for binding to the active site of alcohol dehydrogenase, which reduces the rate at which ethylene glycol is metabolized. In order to be an effective inhibitor of ethylene glycol metabolism, ethanol must be given in large doses to ensure that it is present in high enough concentrations to outcompete ethylene glycol for binding to the active site of alcohol dehydrogenase. Fomepizole is also a competitive inhibitor of alcohol dehydrogenase, but it has a higher affinity for the enzyme than ethanol, which means that it can be given in smaller doses to achieve the same effect. This is why fomepizole is preferred over ethanol as a treatment for antifreeze poisoning.
