IB DP Chemistry -Reactivity 2.1 How much? The amount of chemical change- IB Style Questions For HL Paper 1A -FA 2025
Question
\[C_2H_6(g) + Cl_2(g) \rightarrow C_2H_5Cl(g) + HCl(g)\]
Molar masses: C2H6 = 30.08, Cl2 = 70.90, C2H5Cl = 64.52, HCl = 36.46 g mol−1
(B) 46.6%
(C) 63.9%
(D) 99.9%
▶️ Answer/Explanation
Atom economy = \(\frac{64.52}{30.08 + 70.90} \times 100\)
= \(\frac{64.52}{100.98} \times 100 \approx 63.9\%\)
✅ Answer: (C)
Question
| Option | Limiting reagent | Maximum yield of \( \text{H}_2 \) / mol |
|---|---|---|
| A | HCl | 0.10 |
| B | Mg | 0.20 |
| C | HCl | 0.05 |
| D | Mg | 0.10 |
▶️ Answer/Explanation
The balanced reaction is:
\( \text{Mg}(s) + 2\text{HCl}(aq) \rightarrow \text{MgCl}_2(aq) + \text{H}_2(g) \)
To react completely with \(0.20\) mol of Mg, the required amount of HCl is:
\( 0.20 \text{ mol Mg} \times 2 = 0.40 \text{ mol HCl} \)
But only \(0.10\) mol of HCl is available. Therefore, HCl is the limiting reagent.
The reaction uses HCl and produces \( \text{H}_2 \) in a \(2:1\) ratio:
\( 0.10 \div 2 = 0.05 \text{ mol of } \text{H}_2 \)
Thus, the maximum possible yield of hydrogen is \( \mathbf{0.05\ mol} \).
✅ Answer: (C)
Question
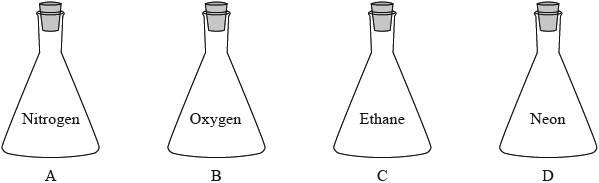
▶️ Answer/Explanation
All containers have the same:
- volume
- temperature
- pressure
Under identical conditions, the number of moles in each container is the same (from the ideal gas law).
Therefore, the container with the largest molar mass will have the greatest total mass.
Molar masses:
- \( M(\text{N}_2) = 28 \)
- \( M(\text{O}_2) = 32 \)
- \( M(\text{C}_2\text{H}_6) = 30 \)
- \( M(\text{Ne}) = 20 \)
Oxygen has the largest molar mass, so its container will have the greatest mass.
✅ Answer: B
