IB DP Chemistry Reactivity 3.3 Electron sharing reactions HL Paper 2- Exam Style Questions - New Syllabus
Question
(a) Explain why a colorimeter set at a wavelength of \(\,500\,\mathrm{nm}\,\) is not suitable to investigate reactions of \(\mathrm{Zn^{2+}}\) compounds. Use data booklet. [\(\,2\,\)]
Inserted data (IB Chemistry Data Booklet): visible region \(\approx 400\text{–}700\,\mathrm{nm}\); green light \(\approx 491\text{–}575\,\mathrm{nm}\). Hence \(\,500\,\mathrm{nm}\,\) is green light in the visible range.
(b) Nitrogen (II) oxide radicals, \(\mathrm{NO\cdot}\), catalyse the decomposition of ozone, \(\mathrm{O_3}\).
(i) Formulate equations showing how \(\mathrm{NO\cdot}\) acts as a catalyst in this reaction. [\(\,2\,\)]
Chlorine also forms free radicals; the bond enthalpy for \(\mathrm{Cl_2}\) is \(\,4.02\times 10^{-19}\,\mathrm{J}\).
(ii) Calculate the minimum frequency of light needed to break this bond. Use data booklet. [\(\,1\,\)]
Inserted data (IB Chemistry Data Booklet): \(E=h\nu\), \(c=\lambda\nu\); Planck constant \(h=6.63\times 10^{-34}\,\mathrm{J\,s}\).
(iii) Calculate the formal charge on each atom in the two Lewis structures of the \(\mathrm{NO_2\cdot\ (g)}\) radical. [\(\,1\,\)]
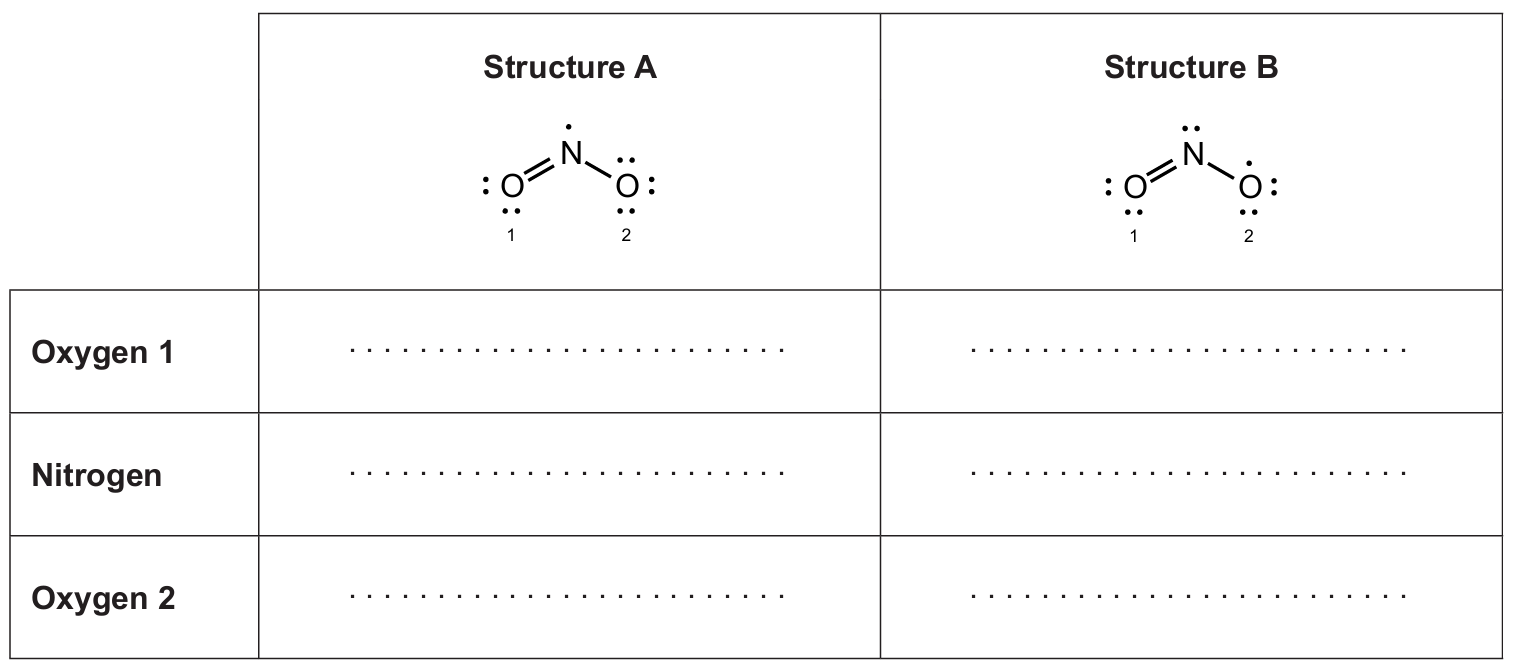
(iv) Lewis structure A is more stable. Suggest, giving one reason, whether the formal charge model supports this. [\(\,1\,\)]
▶️ Answer/Explanation
(a)
\(\mathrm{Zn^{2+}}\) has a \(\mathrm{d^{10}}\) configuration \(\Rightarrow\) no \(\mathrm{d{\leftrightarrow}d}\) electronic transitions in the visible region; its compounds are typically colourless.
A colorimeter set at \(\,500\,\mathrm{nm}\,\) (green, visible) would therefore show \(\approx 0\) absorbance, so it is not suitable to monitor reactions of \(\mathrm{Zn^{2+}}\) compounds.
\(\mathrm{Zn^{2+}}\) has a \(\mathrm{d^{10}}\) configuration \(\Rightarrow\) no \(\mathrm{d{\leftrightarrow}d}\) electronic transitions in the visible region; its compounds are typically colourless.
A colorimeter set at \(\,500\,\mathrm{nm}\,\) (green, visible) would therefore show \(\approx 0\) absorbance, so it is not suitable to monitor reactions of \(\mathrm{Zn^{2+}}\) compounds.
(b)(i)
Catalytic cycle (any acceptable pair):
\(\displaystyle \mathrm{NO\cdot + O_3 \;\rightarrow\; NO_2\cdot + O_2}\)
\(\displaystyle \mathrm{NO_2\cdot + O_3 \;\rightarrow\; NO\cdot + 2\,O_2}\)
(Overall: \(\displaystyle \mathrm{2\,O_3 \rightarrow 3\,O_2}\); \(\mathrm{NO\cdot}\) is regenerated.)
Catalytic cycle (any acceptable pair):
\(\displaystyle \mathrm{NO\cdot + O_3 \;\rightarrow\; NO_2\cdot + O_2}\)
\(\displaystyle \mathrm{NO_2\cdot + O_3 \;\rightarrow\; NO\cdot + 2\,O_2}\)
(Overall: \(\displaystyle \mathrm{2\,O_3 \rightarrow 3\,O_2}\); \(\mathrm{NO\cdot}\) is regenerated.)
(b)(ii)
Given bond enthalpy per bond \(\;E=4.02\times10^{-19}\,\mathrm{J}\). Using \(E=h\nu\):
\(\displaystyle \nu=\frac{E}{h}=\frac{4.02\times10^{-19}}{6.63\times10^{-34}}=6.06\times10^{14}\ \mathrm{Hz}\,\) (minimum frequency).
Given bond enthalpy per bond \(\;E=4.02\times10^{-19}\,\mathrm{J}\). Using \(E=h\nu\):
\(\displaystyle \nu=\frac{E}{h}=\frac{4.02\times10^{-19}}{6.63\times10^{-34}}=6.06\times10^{14}\ \mathrm{Hz}\,\) (minimum frequency).
(b)(iii)

Using \( \mathrm{FC}=\text{valence}-\text{nonbonding}-\tfrac{1}{2}\times\text{bonding\,electrons} \):
Structure A: Oxygen 1 \(=0\), Nitrogen \(=+1\), Oxygen 2 \(=-1\).
Structure B: Oxygen 1 \(=0\), Nitrogen \(=0\), Oxygen 2 \(=0\).
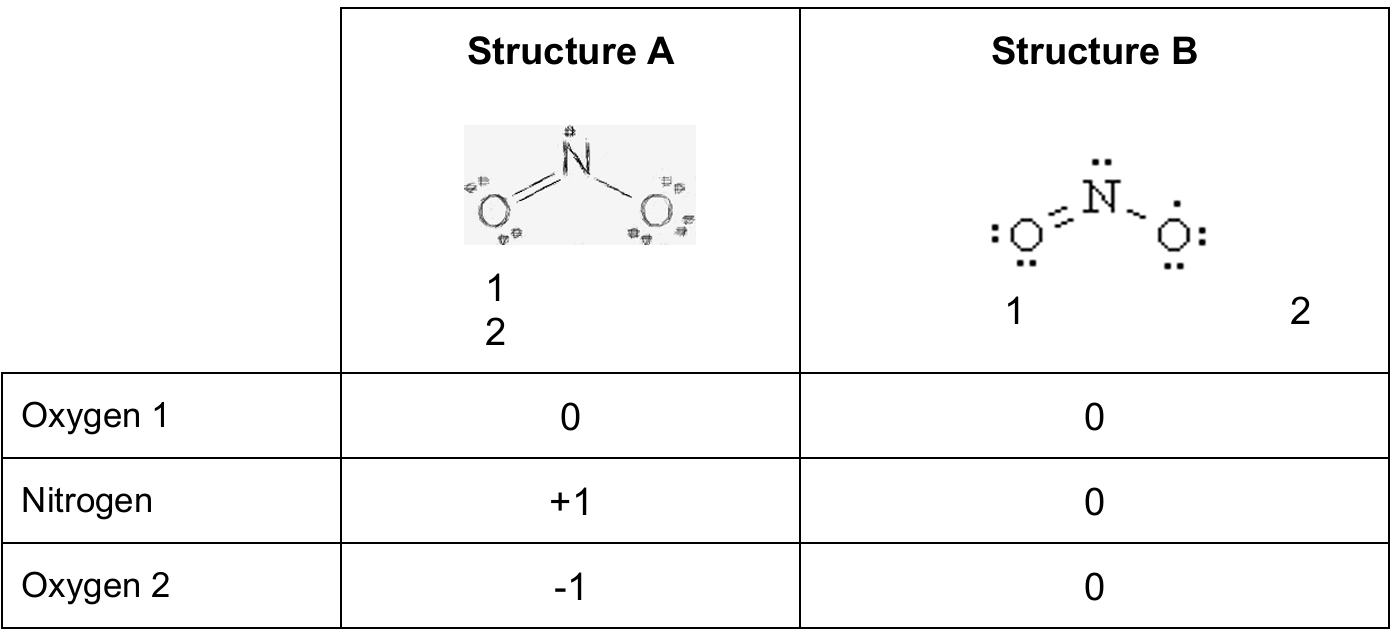
Using \( \mathrm{FC}=\text{valence}-\text{nonbonding}-\tfrac{1}{2}\times\text{bonding\,electrons} \):
Structure A: Oxygen 1 \(=0\), Nitrogen \(=+1\), Oxygen 2 \(=-1\).
Structure B: Oxygen 1 \(=0\), Nitrogen \(=0\), Oxygen 2 \(=0\).
(b)(iv)
No. The formal charge model favours Structure B because all atoms have formal charge \(0\); thus it does not support Structure A being more stable.
No. The formal charge model favours Structure B because all atoms have formal charge \(0\); thus it does not support Structure A being more stable.
