IB DP Chemistry -Reactivity 3.3 Electron sharing reactions - IB Style Questions For SL Paper 2 -FA 2025
Question
(i) Draw the Lewis formula for a molecule of \(\text{HCN}\).
(ii) State and explain the molecular geometry of \(\text{HCN}\).
(iii) \(\text{HCN}\) is a polar molecule. Deduce which atom has a partial positive charge and which atom has a partial negative charge.
(iv) Explain why nitrogen gas, \(\text{N}_2\), has a far lower boiling point than \(\text{HCN}\).
(i) Write an equation to represent this behaviour.
(ii) Outline two ways you could show that a solution was \(0.1 \text{ mol dm}^{-3}\) \(\text{HCN}\) rather than \(0.1 \text{ mol dm}^{-3}\) \(\text{HCl}\).
(iii) Determine the pH of \(0.100 \text{ mol dm}^{-3}\) \(\text{HCN}(aq)\) if \([\text{H}^+]\) is \(7.00 \times 10^{-6} \text{ mol dm}^{-3}\).
\(\text{HCN}(g) + 2\text{H}_2(g) \rightleftharpoons \text{CH}_3\text{NH}_2(g)\)
(i) Write the expression for the equilibrium constant, \(K\).
(ii) Determine the oxidation states that show carbon is reduced in this reaction.
Most-appropriate topic codes (Chemistry):
• TOPIC S2.4: From shape to function — part (a-ii)
• TOPIC S2.3: Intermolecular forces — part (a-iv)
• TOPIC R3.3: Acid and base equilibria — parts (b-i), (b-ii), (b-iii)
• TOPIC R3.1: The equilibrium law — part (c-i)
• TOPIC R2.1: Electron transfer reactions — part (c-ii)
▶️ Answer/Explanation
(a)
(i)
\(\text{H}-\text{C}\equiv\text{N:}\) 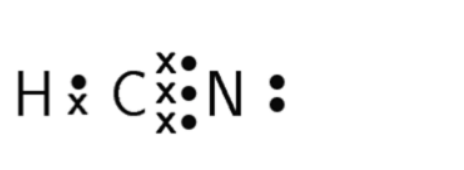
(ii)
• Geometry: Linear.
• Explanation: There are 2 electron domains (bonding pairs) around the central carbon atom and no lone pairs. To minimize electrostatic repulsion, they are oriented \(180^\circ\) apart.
(iii)
• Partial positive (\(\delta^+\)): Hydrogen (H) (or Carbon/C).
• Partial negative (\(\delta^-\)): Nitrogen (N).
(iv)
\(\text{N}_2\) is non-polar and only has weak London (dispersion) forces. \(\text{HCN}\) is polar and possesses stronger dipole-dipole forces (in addition to London forces). The stronger intermolecular forces in \(\text{HCN}\) require more energy to break.
(b)
(i)
\(\text{HCN}(aq) \rightleftharpoons \text{H}^+(aq) + \text{CN}^-(aq)\) OR \(\text{HCN}(aq) + \text{H}_2\text{O}(l) \rightleftharpoons \text{H}_3\text{O}^+(aq) + \text{CN}^-(aq)\).
(ii)
Any two methods:
1. Measure pH: \(\text{HCN}\) will have a higher pH than \(\text{HCl}\).
2. Measure electrical conductivity: \(\text{HCN}\) will have lower conductivity.
3. React with metal/carbonate: \(\text{HCN}\) reacts more slowly.
(iii)
\(\text{pH} = -\log(7.00 \times 10^{-6}) = \mathbf{5.15}\).
(c)
(i)
\[ K = \frac{[\text{CH}_3\text{NH}_2]}{[\text{HCN}][\text{H}_2]^2} \]
(ii)
• Initial Oxidation State: +2 (in \(\text{HCN}\)).
• Final Oxidation State: -2 (in \(\text{CH}_3\text{NH}_2\)).
