IB DP Chemistry - Structure 1.2 The nuclear atom - IB Style Questions For HL Paper 1A -FA 2025
Question
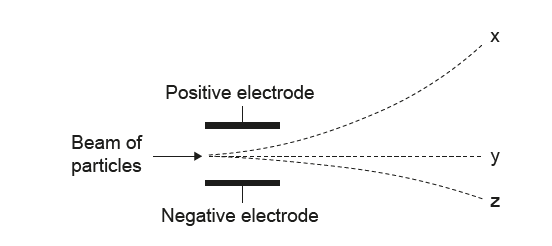
Which option correctly identifies each of the particles?
| Option | Protons | Electrons | Neutrons |
|---|---|---|---|
| A | y | z | x |
| B | x | y | z |
| C | z | x | y |
| D | x | z | y |
▶️ Answer/Explanation
• The path bending strongly downward (toward the positive electrode) must be electrons → x.
• The straight path must be neutrons →y
✅ Answer: (C)
Question
II. The \(M^+\) ion is always the most stable fragment formed during electron bombardment.
III. The \(m/z\) ratio of the \(M^+\) ion peak gives the relative molecular mass of the molecule.
(B) I and III only
(C) II and III only
(D) I, II and III
▶️ Answer/Explanation
Correct option: (B)
Statement I: Correct. The \(M^+\) (molecular ion) peak is not always the most intense peak in a mass spectrum. Often, the base peak (highest intensity) corresponds to a particularly stable fragment ion, not the molecular ion.
Statement II: Incorrect. The \(M^+\) ion is not always the most stable fragment; it can readily break into smaller, more stable ions under electron bombardment.
Statement III: Correct. The \(m/z\) value of the \(M^+\) peak corresponds (for \(z = 1\)) to the relative molecular mass of the original molecule.
Therefore, only statements I and III are correct.
✅ Answer: (B)
Question

(B) \(10.2\)
(C) \(10.5\)
(D) \(10.8\)
▶️ Answer/Explanation
The relative atomic mass \(\left(A_r\right)\) is the weighted average of the isotopic masses using their percentage abundances:
\[ A_r = \frac{80.1 \times 10 + 19.9 \times 11}{100} \]
\[ A_r = \frac{801 + 218.9}{100} = \frac{1019.9}{100} = 10.2 \]
Hence, the relative atomic mass of the element is \(10.2\).
✅ Answer: (B)
