IB DP Chemistry Structure 1.5 Ideal gases HL Paper 2- Exam Style Questions - New Syllabus
Question
Alkanes are commonly occurring organic compounds.
(a) The first four straight chain alkanes are gases at room temperature.
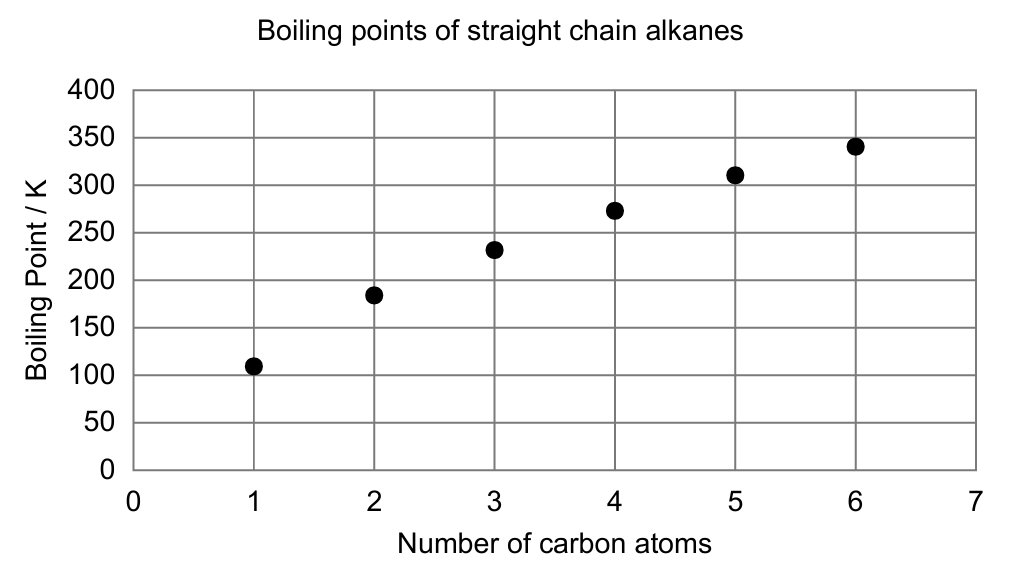
(i) Explain why the boiling point increases from methane to propane. [2]
(ii) Explain why the volume occupied by a sample of propane increases sharply when the sample is heated up from 200 to 250 K at constant pressure. [2]
(iii) Calculate the volume, in dm3, occupied by 6.45 g of propane gas at 100 kPa and 15 °C. [2]
(iv) Outline why the volume occupied by propane (g) at very high pressure is higher than the value calculated using \(PV=nRT\). [2]
(b) Ethane can be converted to chloroethane by reacting with chlorine gas, Cl2 (g), in the presence of UV light.
State the type of reaction and the name of the mechanism by which it occurs. [1]
State the type of reaction and the name of the mechanism by which it occurs. [1]
(c) Chloroethane can be converted to ethanol. Identify the reagent and conditions necessary for this reaction to occur. [2]
Reagent: __________
Conditions: __________
▶️ Answer/Explanation
Markscheme (with detailed working)
(a)(i)
London (dispersion) forces between molecules increase from CH4 to C3H8 because the electron cloud/number of electrons and surface area increase, so intermolecular attractions are stronger and a higher temperature is needed to boil. (M1) A1
London (dispersion) forces between molecules increase from CH4 to C3H8 because the electron cloud/number of electrons and surface area increase, so intermolecular attractions are stronger and a higher temperature is needed to boil. (M1) A1
(a)(ii)
On heating from 200 K to 250 K at constant pressure, the sample boils/vaporizes; molecules gain sufficient energy to overcome intermolecular forces. The gas occupies far more volume than the liquid (much lower density), so the volume rises sharply. (M1) A1
On heating from 200 K to 250 K at constant pressure, the sample boils/vaporizes; molecules gain sufficient energy to overcome intermolecular forces. The gas occupies far more volume than the liquid (much lower density), so the volume rises sharply. (M1) A1
(a)(iii)
Molar mass \(M_r(\mathrm{C_3H_8}) = 44.11\). Mass \(m = 6.45~\mathrm{g}\).
\[ n = \frac{m}{M_r} = \frac{6.45}{44.11} = 0.146~\text{mol} \] Use the ideal gas equation \(PV=nRT\) with \(P=100~\text{kPa}\), \(T=15^\circ \text{C}=288~\text{K}\), \(R=8.31~\text{J\,K}^{-1}\text{mol}^{-1}\) (note \(1~\text{J}=1~\text{kPa\,dm}^3\)).
\[ V=\frac{nRT}{P}=\frac{(0.146)(8.31)(288)}{100}=3.49~\text{dm}^3 \quad(\text{to }3\,\text{s.f.}) \] \(\boxed{V \approx 3.49~\text{dm}^3}\) (allow \(3.49\text{–}3.59\ \text{dm}^3\)). (M1) A1
Data used (Chemistry Data Booklet): \(PV=nRT\) (Section 1) and \(R=8.31~\mathrm{J\,K^{-1}\,mol^{-1}}\)
(a)(iv)
Real gases deviate from ideal behaviour at very high pressure: ideal gas model assumes particles have no volume, but propane molecules have finite size so the measured volume is larger than \(V\) from \(PV=nRT\). (M1) A1
Real gases deviate from ideal behaviour at very high pressure: ideal gas model assumes particles have no volume, but propane molecules have finite size so the measured volume is larger than \(V\) from \(PV=nRT\). (M1) A1
(b)
Type of reaction: substitution; mechanism: free radical (radical halogenation). A1
Type of reaction: substitution; mechanism: free radical (radical halogenation). A1
(c)
Reagent: \(\mathrm{NaOH}\) (hydroxide, \(\mathrm{OH^-}\)).
Conditions: warm/heat (often reflux) with aqueous solution (accept aprotic solvent alternatives).
A1 A1
Reagent: \(\mathrm{NaOH}\) (hydroxide, \(\mathrm{OH^-}\)).
Conditions: warm/heat (often reflux) with aqueous solution (accept aprotic solvent alternatives).
A1 A1
Total Marks: 9
