1.3 Reacting masses and volumes
Essential Idea:
Mole ratios in chemical equations can be used to calculate reacting ratios by mass and gas volume
Understandings:
- Reactants can be either limiting or excess.
- The experimental yield can be different from the theoretical yield.
- Avogadro’s law enables the mole ratio of reacting gases to be determined from volumes of the gases.
- The molar volume of an ideal gas is a constant at specified temperature and pressure.
- The molar concentration of a solution is determined by the amount of solute and the volume of solution.
- A standard solution is one of known concentration.
Applications and Skills:
- Solution of problems relating to reacting quantities, limiting and excess reactants, theoretical, experimental and percentage yields.
- Calculation of reacting volumes of gases using Avogadro’s law.
- Solution of problems and analysis of graphs involving the relationship between temperature, pressure and volume for a fixed mass of an ideal gas.
- Solution of problems relating to the ideal gas equation.
- Explanation of the deviation of real gases from ideal behaviour at low temperature and high pressure.
- Obtaining and using experimental values to calculate the molar mass of a gas from the ideal gas equation.
- Solution of problems involving molar concentration, amount of solute and volume of solution.
- Use of the experimental method of titration to calculate the concentration of a solution by reference to a standard solution.
1.3 The Limiting Reagent
- In a reaction, we can describe reactants as being ‘limiting’ or in ‘excess’.
Limiting reagent– this is the reactant that gets completely used up in the reaction.
Excess – reactant that is leftover after the reaction
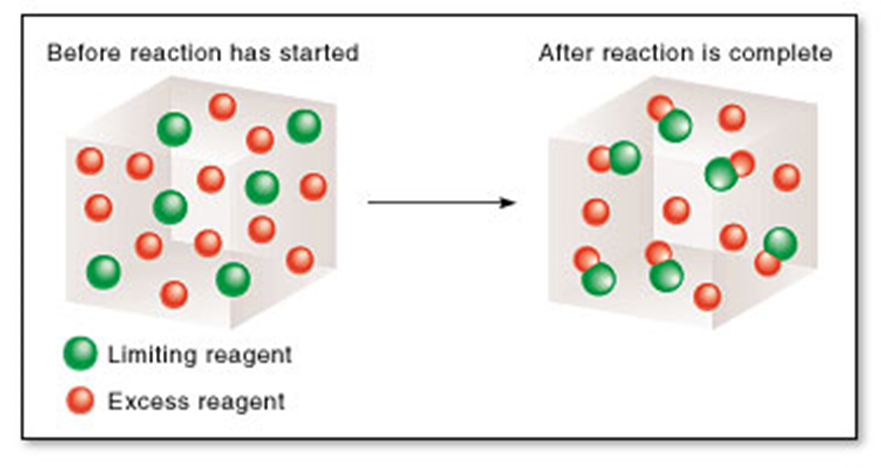
Application of Limiting Reactant
- The concept of limiting reactant is often useful in the design of experiments and synthetic processes.
- By deliberately making one reactant available in an amount greater than that determine by its mole ratio in the balanced equation, it ensures that the other reactant is limiting and will be fully used up.
The Limiting Reagent (LR)
- Consider the reaction below:
2 H2 + O2 → 2 H2O
- Determine the limiting reactant and the maximum moles of water produced if 2.0 mol H2 reacts with 2.0 mol O2.
–Set up simple ratio calculation to determine which reactant produces LESS water – this will be the LR.
2.0 H2 * (2 H2O / 2 H2) = 2 mol H2O produced (Maximum amount of water that can be produced)
H2 is LR
2.0 O2 * (2 H2O / 1 O2) = 4 mol H2O produced
Theoretical Yield vs. Actual (Experimental) Yield
- What is the actual (experimental) yield?
- Amount of product that is actually isolated at end of reaction (amount obtained experimentally).
- For many experiments actual yield is much less than theoretical.
- This may be due to errors, mistakes, side reactions, contamination, etc.
How do you calculate percent yield?
- %\(Yield = \frac{Actual}{Theoritical}\times 100\)
- In the previous example, for 18.1 g NH3 and 90.4 g CuO, the theoretical yield of Cu was 72.4 g.
- If the actual yield is 58.3 g Cu, what is the percent yield?
- %Yield = (58.3/72.4) x 100= 80.5%
Molar Volume of a Perfect Gas
- The distance between gas particles is much larger than the size (volume) of the particles.
- Gas particles have negligible forces between them.
- The chemical nature of a gas is irrelevant to its volume.
–The blue particle is twice the size of the red particle, but the blue particles are not taking up twice the amount of space.
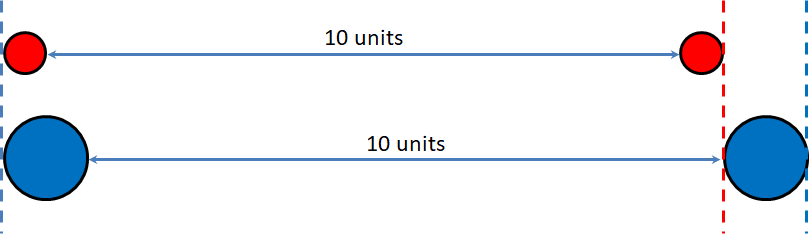
Avogadro’s Law & Molar Volume
Avogadro’s Law states that equal volumes of gases, when measured at the same temperature and pressure, contain an equal number of particles.
- Mathematically, Avogadro’s law is:
V α n (V is proportional to the number of moles for any gas at constant T and P)
V/n = k (k is a constant)
–V is the volume of the gas, k is a proportionality constant, and n is the number of moles of gas particles (volume is directly proportional to the number of moles of gas at STP, regardless of the type of gas!).
Standard Temperature and Pressure:
(DATA BOOKLET)
- Temperature (T) = 273 K
- Pressure (P) = 100 kPa
- Molar Volume of Gas (1 mole of any gas at STP) = 2.27 x 10-2 m3 mol-1 or 22.7 dm3 mol-1
Gas Laws
- 4 Physical Properties of Gases:
- Pressure, P
- Volume, V
- Temperature, T
- Amount = moles
Boyles Law (Pressure & Volume)
- All experiment were conducted at constant T and constant # of moles (n) of gas.
- How are volume and pressure related?
–Inversely, as P ↑, V ↓
- PV = k
–(k is a constant)
- P1V1 = P2V2
Charles’s Law
- Charles worked on relationship of how volume changes with temperature.
- Pressure (P) and moles (n) constant during experiments.
- How are V & T related?
–Directly
–V ↑ as T ↑
–T is measured in Kelvin
Gay-Lussac’s Law
- Worked on the relationship between pressure (P) and temperature (T).
- Volume (V) and moles (n) kept constant.
- How are P and T related?
–P is directly proportional to Kelvin T.
The Ideal Gas Law
Boyle’s Law, Charles’ Law, Gay-Lussac’s Law, and Avogadro’s Law
- If we combine the experimental laws above we obtain the ideal gas law:
PV= nRT
Where:
- P = pressure V = Volume
- n = Moles R = Universal Gas Constant
- T = Temperature in Kelvin
Real Gases vs. Ideal Gases
- An ideal gas is one that follows ALL the assumptions of kinetic molecular theory (KMT).
–Gases are in constant random motion
–Gas volume is negligible
–Intermolecular forces are insignificant
- Under certain temperature and pressure conditions, the assumptions of KMT break down.
–Real gases deviate from an ideal gas.
Components of a Solution
- Example: NaCl (aq)
–What is the solvent?
- Water
–What is the solute?
- NaCl
MOLE
ATOMIC MASS
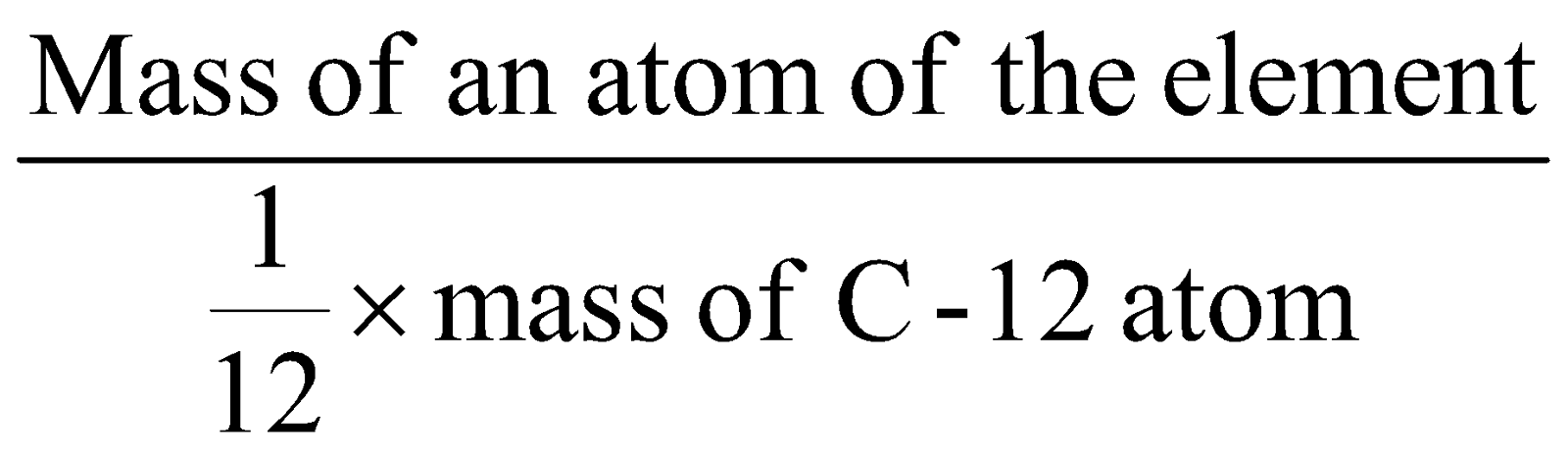
AVERAGE ATOMIC MASS
DETERMINATION OF ATOMIC MASS
- Dulong and petit’s rule : It is based on experimental facts. “At ordinary temperature, product of atomic mass and specific heat for solid elements is approximately 6.4 and this product is known as atomic heat of the element”
- Specific heat method : This method is for gases.
 , where Cp = specific heat at constant pressure and Cv = specific heat at constant volume. the ratio g is a constant = 1.66 for monoatomic, 1.40 for diatomic, 1.33 for triatomic gas and atomic mass of gaseous element
, where Cp = specific heat at constant pressure and Cv = specific heat at constant volume. the ratio g is a constant = 1.66 for monoatomic, 1.40 for diatomic, 1.33 for triatomic gas and atomic mass of gaseous element
- Chloride formation method : This method converts the element (whose mass is to be determined) into volatile chloride whose vapour density is found by Victor Mayer method.
- Vapour density method is suitable for elements having volatile chlorides.
- Mitscherlich’s law of isomorphism : It states that isomorphous substances have similar chemical constitution. Isomorphous substances form crystals of same shape and valencies of elements forming isomorphous salts are also same. eg: ZnSO4. 7H2O, MgSO4.7H2O and FeSO4.7H2O are isomorphous.
GRAM ATOMIC MASS (GAM)
MOLECULAR MASS :
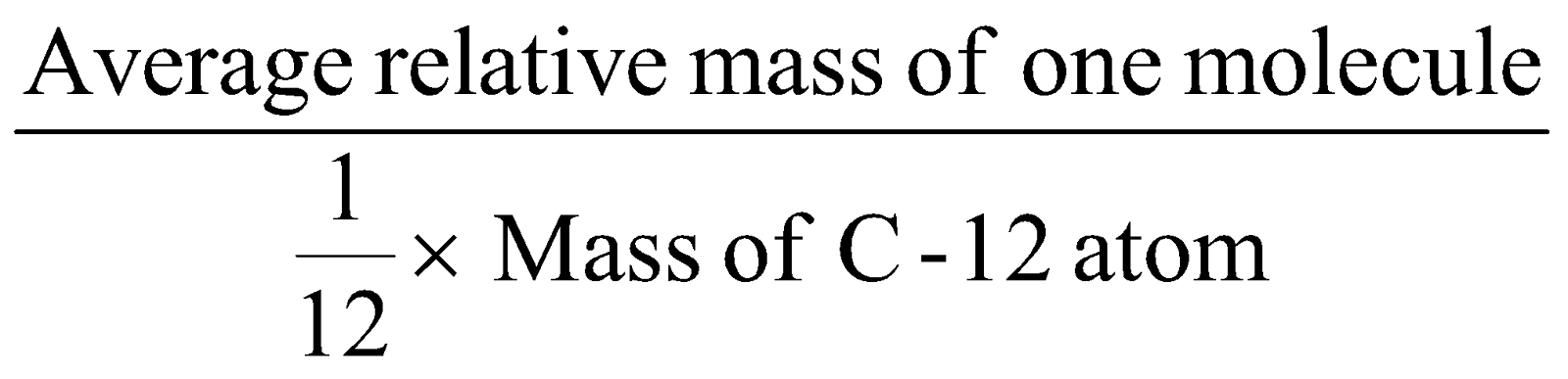
CALCULATION OF MOLECULAR MASS :
- Graham’s law of diffusion : It states that rate of diffusion of two gases is inversely proportional to the square root of ratio of their molecular weights.
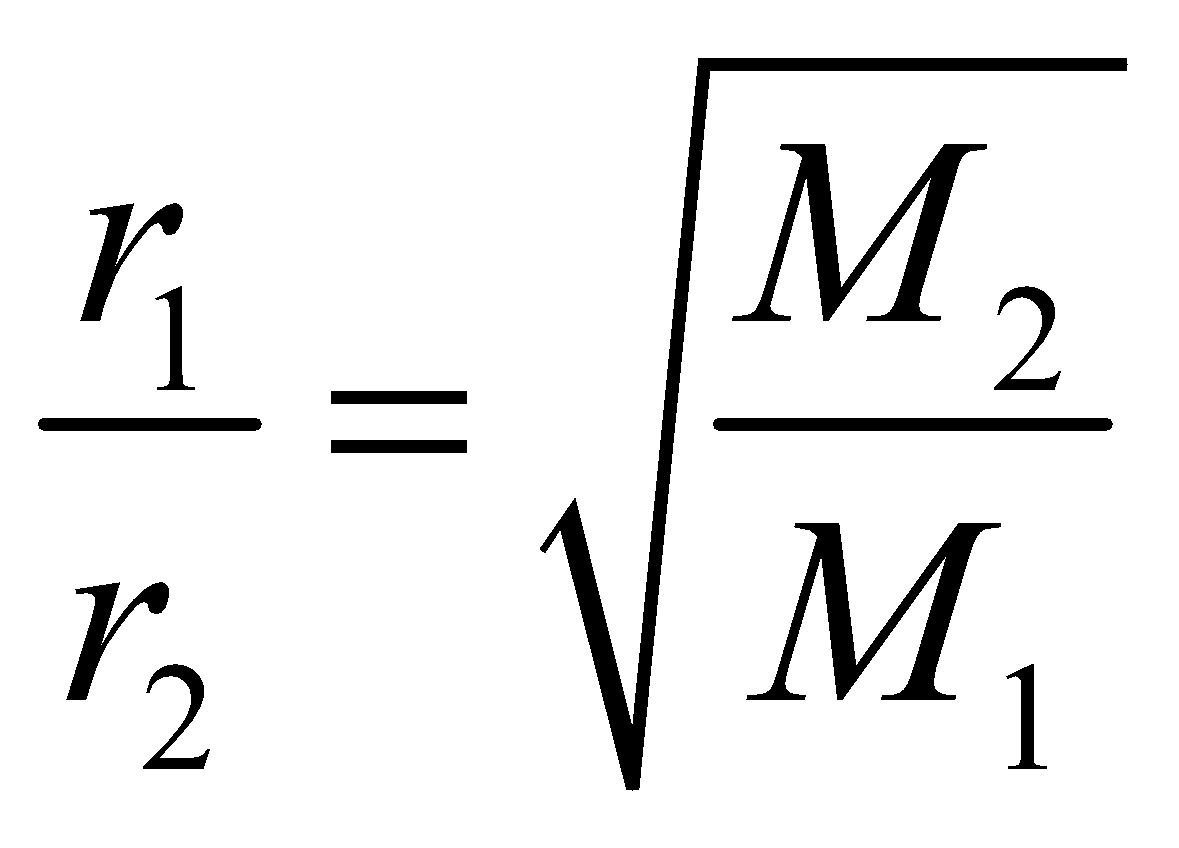
- Victor meyer method : This method can determine the molecular mass as
- Vapour density method : Vapour density is the ratio of volume of a gas to the mass of same volume of hydrogen under identical conditions.
- Colligative properties method : This method can be helpful in determining molecular mass as
GRAM MOLECULAR MASS OR MOLAR MASS :
EQUIVALENT MASS :
- Equivalent mass for elements =

- Equivalent mass for acids =

- Equivalent mass for bases =

- Equivalent mass for salts =

- Equivalent mass for oxidising agents =

- Equivalent mass for reducing agents =

- Equivalent weight of radicals =

FORMULA MASS :
ACIDITY :
BASICITY :
GRAM EQUIVALENT MASS (GEM) :
METHODS OF DETERMINING EQUIVALENT MASSES :
- Hydrogen displacement method : It is for metals which can displace H2 from acids.
- Metal displacement method : It utilises the fact that one GEM of a more electropositive metal displaces one GEM of a less electropositive metal from its salt
 .
. - Conversion method : When one compound of a metal is converted to another compound of similar metal then
- Electrolytic method :
It states that the quantity of substance that reacts at electrode when Faraday of electricity is passed is equal to its GEM.
- Oxide method :
- Double decomposition :
- Neutralisation method for acids and bases :
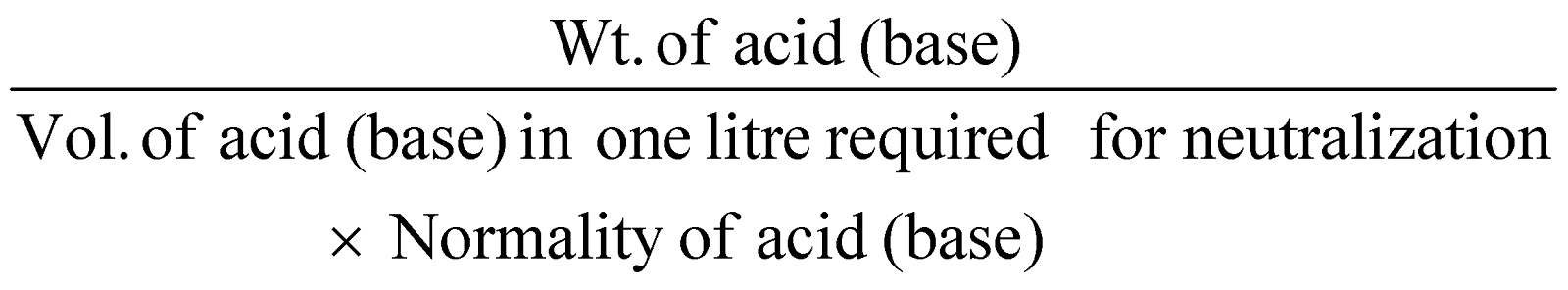
- Silver salt is method commonly used for organic acids.
- Platinichloride method for bases :
- Chloride method :
- Volatile chloride method :
CHEMICAL EQUATION :
- An equation which has not been equalised in terms of number of atoms of reactants and products is called a skeleton equation.
- An equation having equal number of atoms of various kinds on both sides is a balanced equation.
