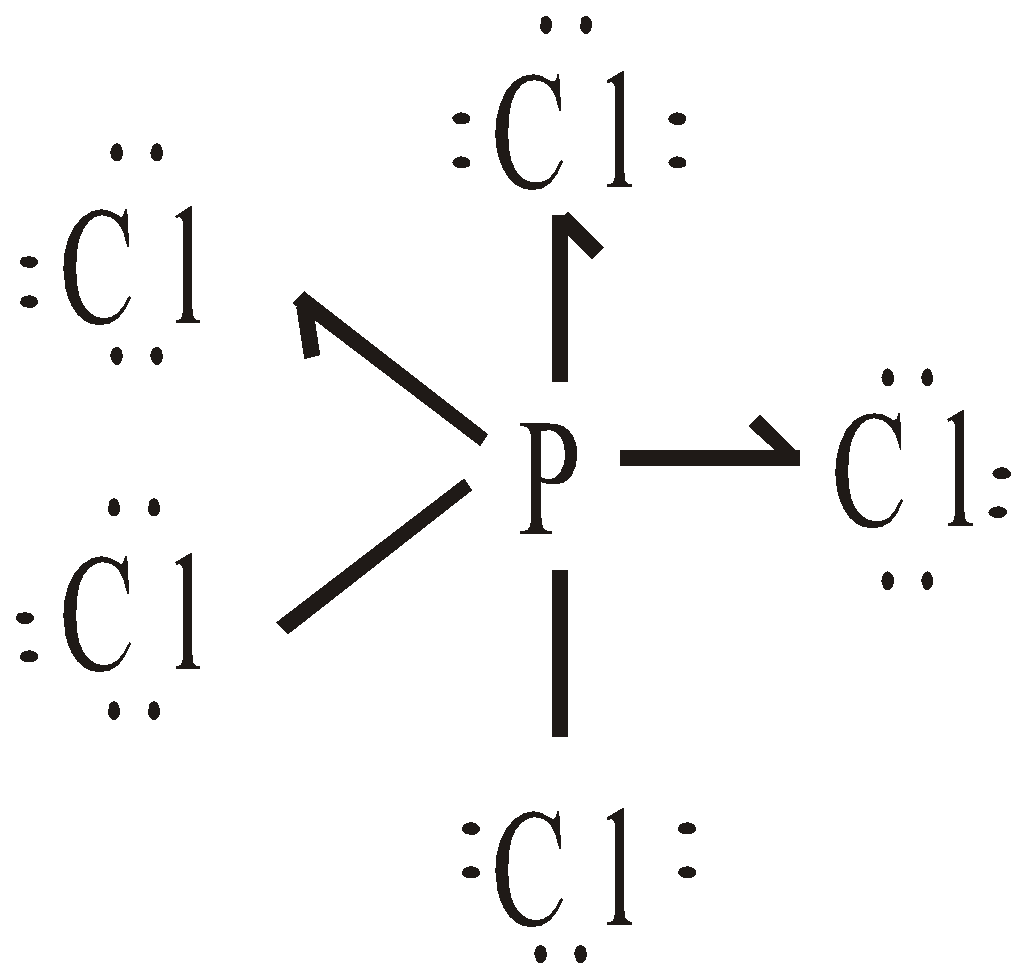4.2 Covalent bonding
Essential Idea:
Covalent compounds form by the sharing of electrons.
Understandings:
- A covalent bond is formed by the electrostatic attraction between a shared pair of electrons and the positively charged nuclei.
- Single, double and triple covalent bonds involve one, two and three shared pairs of electrons respectively.
- Bond length decreases and bond strength increases as the number of shared electrons increases.
- Bond polarity results from the difference in electronegativities of the bonded atoms.
Applications and Skills:
- Deduction of the polar nature of a covalent bond from electronegativity values.
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CHEMICAL BOND
A chemical bond is a sort of attraction which keeps the two atoms together. Thus depending upon the variety of force, there may be variety of chemical bonds.
REASONS FOR THE FORMATION OF A CHEMICAL BOND
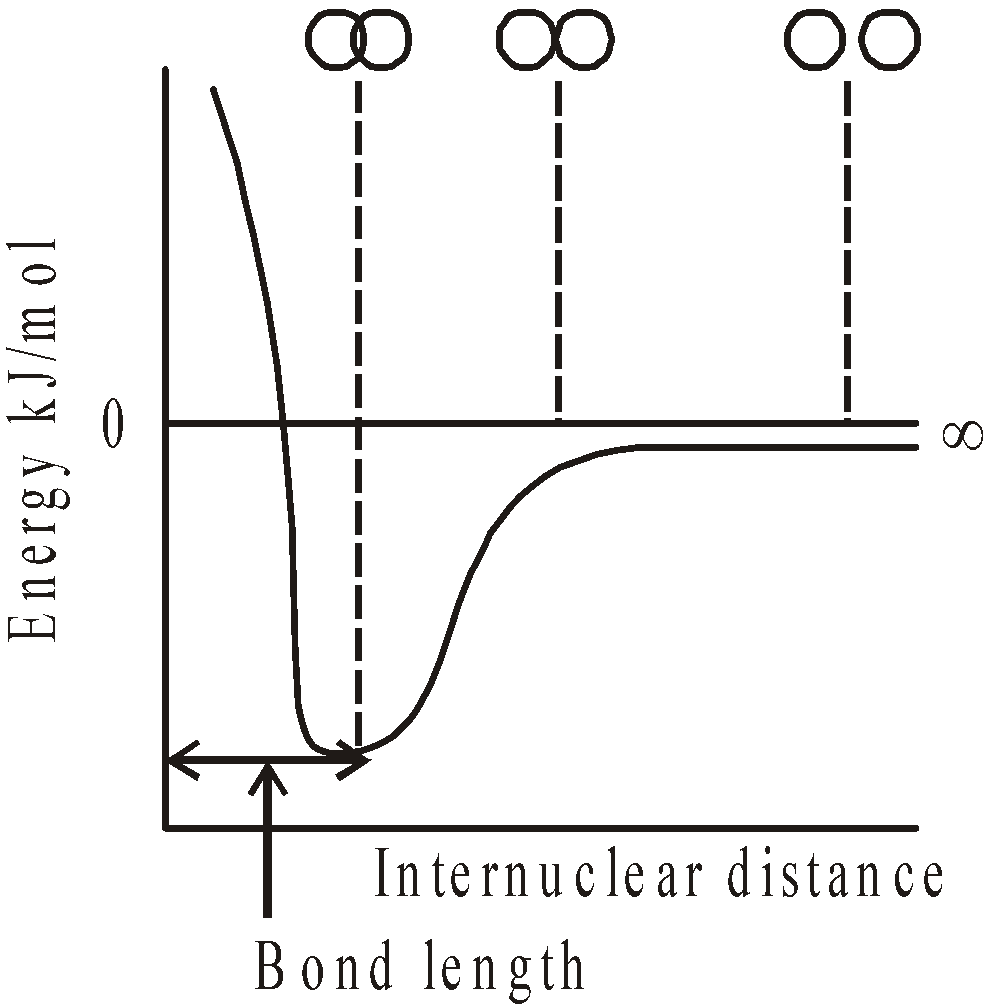
- Energy concept : When two atoms approach each other, the attractive and repulsive forces operate between them. The distance at which the attractive forces overweigh the repulsive forces is known as the bond distance, the potential energy of the system is minimum and the bond is said to be formed.
- Lewis and Langmuir (Octet Rule) : Concept of stable electronic configuration.
Atoms enter into chemical bonding to acquire the stable inert gas electronic configuration. They can do so by losing, gaining or sharing of electrons.
Lewis symbols : The electrons present in the outermost energy level of an atoms and known as valence electrons. Only valence electrons are involved in the combination of two atoms. The representation of valence electrons on an atom called Lewis Symbol eg-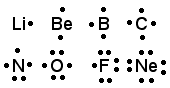
TYPES OF CHEMICAL BOND : (BY KOSSEL AND LEWIS)
Depending upon the mode of acquiring the stable electronic configuration, the chemical bonds may be
- Ionic or electrovalent bond
- Covalent bond
- Coordinate or dative bond
- Metallic bond
- Hydrogen bond
- Weak van der waal’s forces of attraction
IONIC BOND (BY KOSSEL AND LEWIS)
Ionic bond is formed by the complete transference of one or more valence electrons of one atom to the valence shell of the other atom. Both atoms are converted into ions and have the electronic configuration of nearest noble gases. The electrostatic attraction between these oppositely charged ions, which always tends to decrease the potential energy of the system is known as the ionic bond. Consider the formation of KCl. The electronic configuration of K, Cl and their ions (K+ and Cl–) are given below
K (19) 1s2, 2s2, 2p6, 3s2, 3p6, 4s1
K+(18) 1s2, 2s2, 2p6, 3s2, 3p6 Inert gas (Ar) configuration
Cl (17) 1s2, 2s2, 2p6, 3s2, 3p5
Cl– (18) 1s2, 2s2, 2p6, 3s2, 3p6 Inert gas (Ar) configuration
K+ +Cl–  KCl
KCl
The number of electrons lost or gained by an atom represent the electrovalency of the atom.
FACTORS AFFECTING THE FORMATION OF IONIC BOND
There are three main factors
- Ionisation energy (I) : The lower the value of the ionisation energy of an atom greater will be the ease of formation of cation from it.
Note: the size of the cation is always smaller than the atom from which it is derived
- Electron affinity (E) : The higher the electron affinity of an atom the greater will be the ease of formation of anion from it.
The size of the anion is always larger than the atom from which it is derived. - Lattice structure : The electrostatic field of cations and anions extends in all directions, their union is not limited to form a single molecule, rather a cluster of ions, having three dimensional orderly arrangement, known as the lattice structure is formed.
- Lattice energy (U) : The amount of heat evolved when one mole of ionic compound is formed from positive and negative ions in the crystalline form is known as the lattice energy.
BORN HABER CYCLE
The formation of ionic compounds in terms of energy can be seen by Born Haber cycle eg. formation of NaCl is as follows
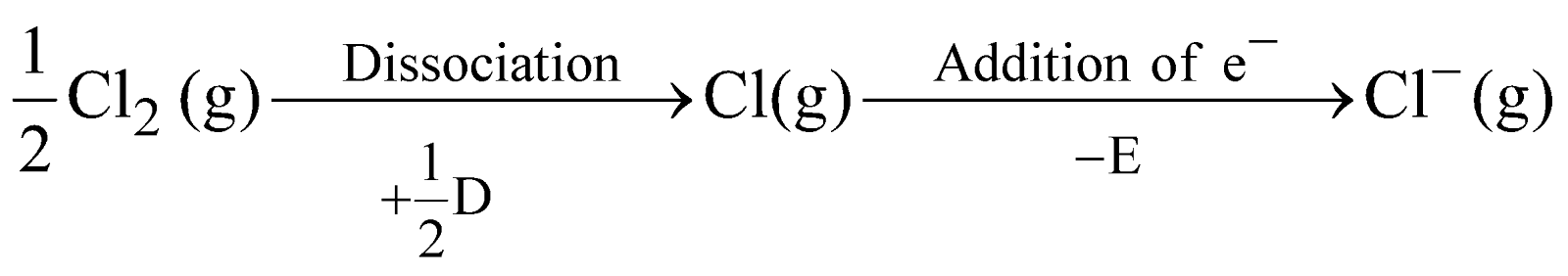
where S = Heat of sublimation of Na metal
I = Ionisation energy of Na
D = Heat of dissociation of molecular chlorine
E = Electron affinity of chlorine atom
U = Lattice energy of NaCl
The amount of heat liberated (Q) in the overall reaction is the heat of formation of NaCl.
Thus Q = S + I + 1/2 D – E – U
(The negative sign indicates heat is evolved or liberated)
The important energy terms are I, E and U. The more the negative value of Q, the greater will be the stability of ionic compound. Hence formation of ionic compound is favoured by:-
- Low ionisation energy (I) of the metal
- High electron affinity (E) of the non-metal.
- Higher lattice energy (U) of the compound
In general the alkali metals have low values of ionisation energy and halogens have high values of electron affinity. Hence they form the stable ionic compounds.
FACTORS AFFECTING LATTICE ENERGY (U)
The force of attraction between two oppositely charged ions is given by well known Coulomb’s law as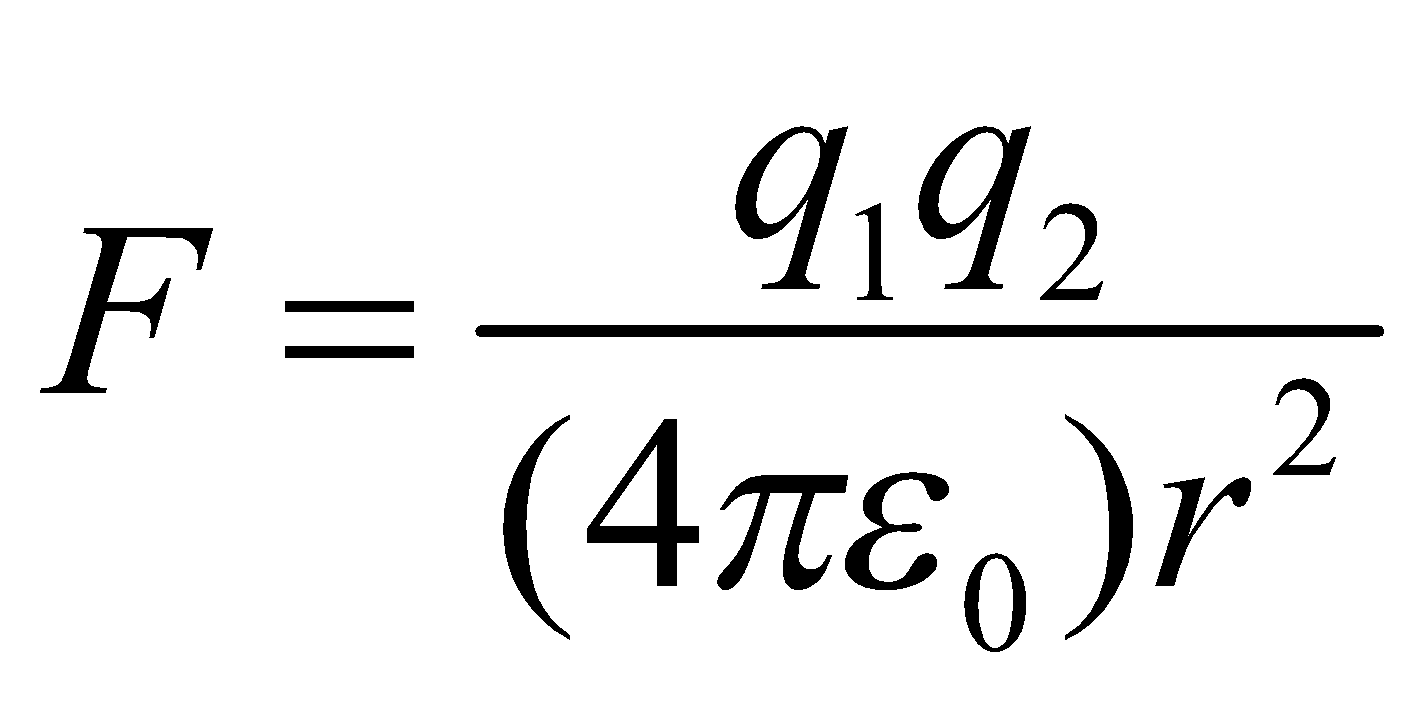 ,
,
Where q1 and q2 are the charges of ions in coulombs, is the permittivity factor and r is the distance between ions. Hence lattice energy depends upon
- Charge on ions : The higher the charge on ions, the greater will be the force of attraction between them and greater will be the lattice energy. The order of lattice energy for different solids is as follows
bi – bivalent > uni – bivalent > uni – univalent
solids solids solids
MgO > CaCl2 > NaCl
- Size of ions : The smaller the size of the ions, the lesser will be distance between them, the greater will be the force of attraction and so greater will be the lattice energy.
FAJAN’S RULE
This rule is for covalent character of an ionic bond. Covalent character of an ionic bond is favoured by
- Small positive ion
- Large negative ion
- Large charge on ions
Thus for a fixed cation, the larger the size of anion, the more the magnitude of the charge, the more is covalent character eg. covalent character of sodium halides follows the order
NaI > NaBr > NaCl > NaF
For fixed anion, the smaller the size of cation, the more the magnitude of the charge, the more is the covalent character eg.
BeCl2 > MgCl2 > CaCl2 > SrCl2 > BaCl2
It has been observed that a cation having 18 (s2p6d10) electrons in outermost shell (pseudo noble gas configuration) can polarise the anion more than cation having 8 (s2p6) noble gas electronic configuration. Hence CuCl is more covalent than NaCl. Similarly AgCl is more covalent than KCl.
POLARISING POWER OF CATION
It is generally represented by and also known as ionic potential charge density. It can be represented as
POLARISATION
When cation and anion come closer to each other, the electron cloud of anion is attracted towards the cation, some partial sharing of electrons take place, the anion is distorted and the effect is known as polarisation.
More the effect of polarisation, more is the sharing of electrons and the more is covalent character of ionic bond.
PROPERTIES OF IONIC COMPOUNDS
The important properties are as follows
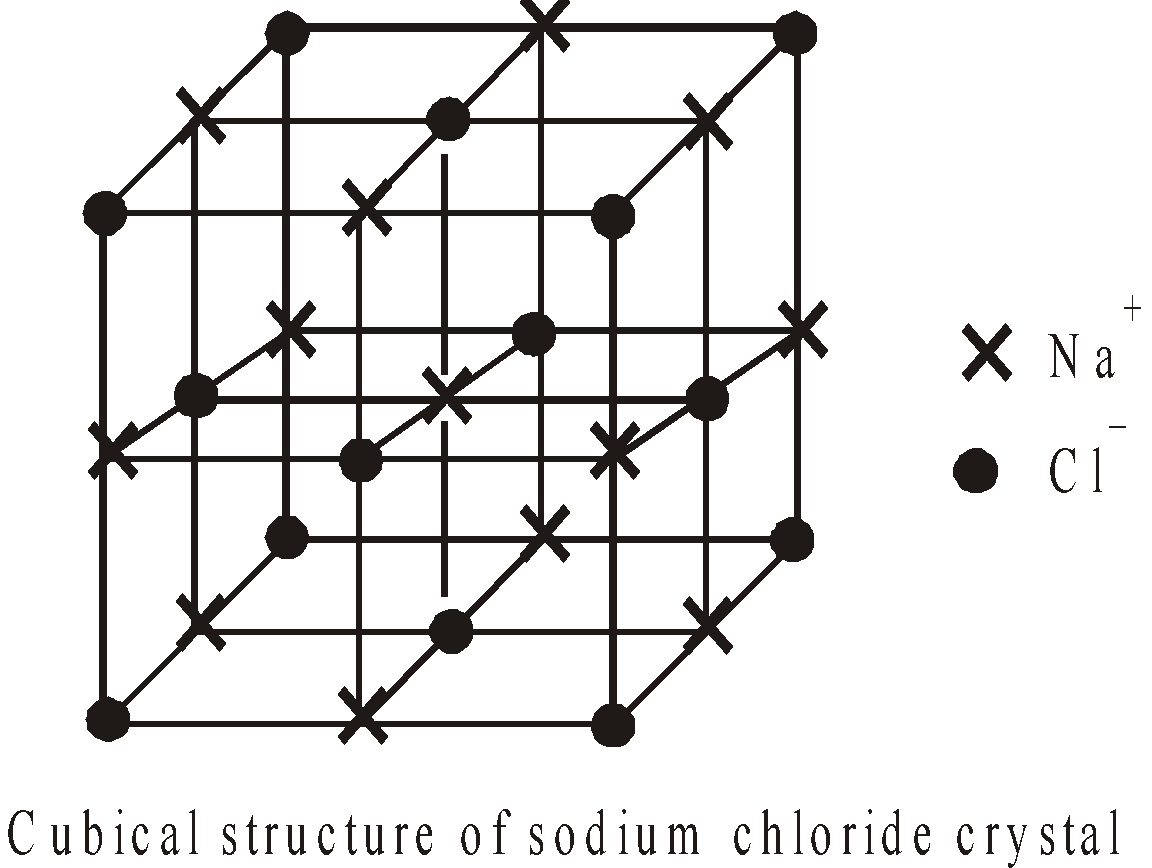
- Crystal structure : The crystalline ionic compounds have well defined crystal structure or crystal lattice eg in NaCl each Na+ is surrounded by six Cl– ions and vice versa
- Melting and boiling points : Ionic compounds have high m.pt and b.pt due to powerful electrostatic force between ions.
- Solubility : They are generally soluble in polar solvents (having high value of dielectric constant). The solubility of ionic compounds decreases with increase in covalent character of ionic compounds. It is also governed by
- Lattice energy : More the lattice energy, lesser is the solubility, eg. sulphates and phosphates of Ba and Sr are insoluble in water due to high lattice energy.
- Heat of hydration : More the heat of hydration, more is the solubility. eg AlCl3 though covalent in nature is soluble in water due to high value of heat of hydration.
- Electrical conductivity : In solid state they do not conduct electricity since there is no free movement of electrons but in molten state and in solution they conduct electricity.
- Isomorphism : NaF and MgO are isomorphous due to similar electronic structure
Similarly K2S and CaCl2 are isomorphous
K+ S2- K+ Cl– Ca++ Cl–
2,8,8 2,8,8 2,8,8 2,8,8 2,8,8 2,8,8
VARIABLE ELECTROVALENCY
It is due to unstable atomic core and inert pair effect. When valence electrons from a metal atom are removed it leaves behind the atomic core or atomic kernel. The latter with 2 or 8 electrons are stable. In case of transition elements the atomic cores are not very stable. They may lose one or more electrons thus exhibiting variable electrovalency eg. iron and copper etc.
Fe (2, 8, 14, 2) 1s2, 2s2p6, 3s2p6 d6, 4s2
Fe++ (2, 8, 14) 1s2, 2s2p6, 3s2p6 d6
Fe+++ (2, 8, 13) 1s2, 2s2p6, 3s2p6 d5
The energy difference between 4s and 3d subshell is not very large.
INERT PAIR EFFECT
The reluctance of s electron pair of some heavy elements
(p – block elements present at the bottom of group) to take part in bonding is known as inert pair effect. Thus Tl (6s2, 6p1) show +1 electrovalence. Similarly Sn(5s2p2) and Pb (6s2p2) show + 2 oxidation state commonly. In such elements the lower oxidation state is more stable than higher oxidation state.
(p – block elements present at the bottom of group) to take part in bonding is known as inert pair effect. Thus Tl (6s2, 6p1) show +1 electrovalence. Similarly Sn(5s2p2) and Pb (6s2p2) show + 2 oxidation state commonly. In such elements the lower oxidation state is more stable than higher oxidation state.
COVALENT BOND : (BY LEWIS AND LANGLEY)
According to G. N. Lewis atoms may also combine by sharing of electrons present in their outermost shells and attain noble gas electronic configuration. One shared pair of electrons constitute a single bond, two electron pairs constitute a double bond and so on. The bonds thus formed ore known as covalent bonds.
COVALENCY
It is the number of electron pairs shared by one atom of the element in combination with other atoms in a molecule.
Variable Covalency
Generally the covalency of an element is equal to the total number of unpaired electrons in s– and p– orbitals of the valence shell. For example
The variable covalency is shown by elements having vacant d-orbitals in their valence shell. The unpairing of the s- and p- electrons is possible by promoting them to d- orbitals. For example
Ground state Covalency of sulphur = 2
First excited state Covalency of sulphur = 4
= 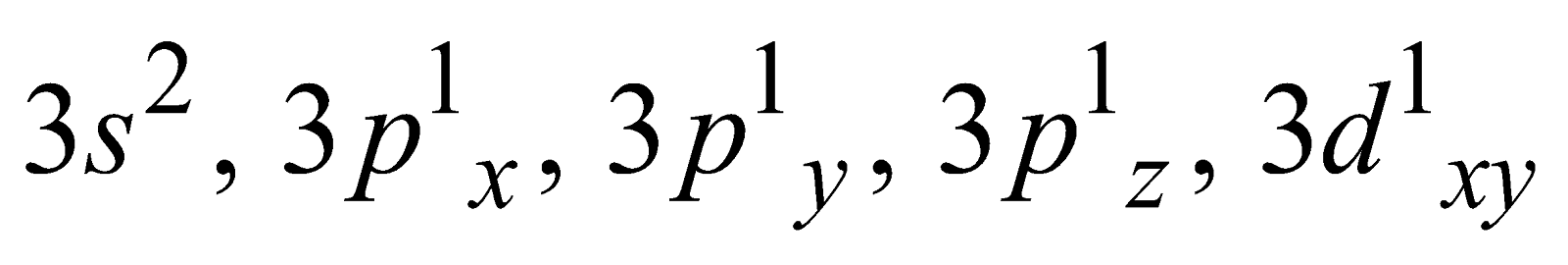
Second excited state Covalency of sulphur = 6
= 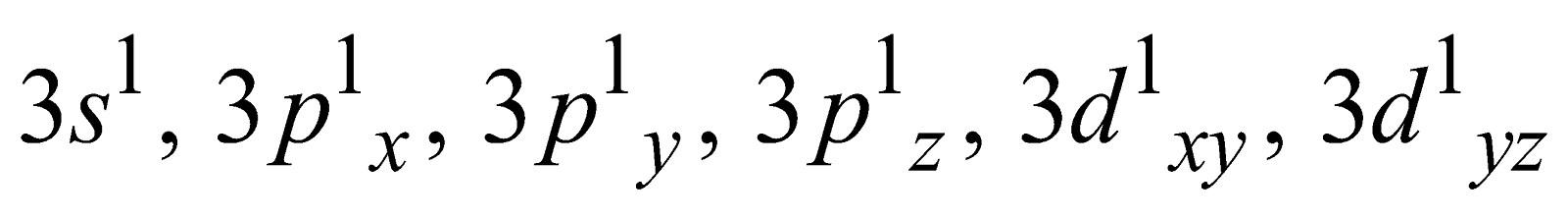
Similarly, other than fluorine, the halogens have high covalencies equal to 3, 5 and 7. Phosphorous shows covalencies equal to 3 and 5.
The elements having no d-orbitals do not exhibit variable covalency
EXAMPLES OF COVALENT COMPOUNDS
- Chlorine (Cl2)
- Carbon dioxide (CO2)
- Nitrogen (N2)
NATURE OF COVALENT BOND
The nature of covalent bond is explained by
(i) Heitler – london theory Valence bond theory
(ii) Pauling – slater theory
(iii) Hund – mulliken theory } Molecular orbital theory
VALENCE BOND THEORY
A covalent bond is formed by the overlapping between two half filled atomic orbitals having electrons with opposite spin.
- Sigma bond (σ – bond) – The following overlappings result in the formation of sigma bond
- s – s overlapping
- s – p overlapping
- p – p head on overlapping
- pi – bond (
 – bond) – It is formed by the sidewise or lateral overlapping between unhybridised p – atomic orbitals
– bond) – It is formed by the sidewise or lateral overlapping between unhybridised p – atomic orbitals
p – p side wise or lateral overlapping.
- π-bond is a weaker bond than σ – bond.
- The strength of bond depends upon the extent of overlapping between atomic orbitals. Greater the overlapping stronger the σ – bond is. It follows the following order
s – s > s – p > p – p
- Single bond is always σ bond.
- In multiple bonds only one is σ bond, others are π – bonds
- π- bond is not formed by hybrid orbitals.
LIMITATIONS OF VALENCE BOND THEORY
- It fails to explain the magnetic properties of some molecules
- Bonding in electron deficient compounds.
MOLECULAR ORBITAL THEORY OR HUND- MULLIKEN THEORY
According to this theory the atomic orbitals combine to form the molecular orbitals. The number of molecular orbitals formed are equal to the number of atomic orbitals involved and they belong to the molecule.
- The molecular orbitals are formed by LCAO method (linear combination of atomic orbitals) i.e. by addition or subtraction of wave functions of individual orbitals thus
- Molecular orbital of lower energy is known as bonding molecular orbital and of higher energy is known as antibonding molecular orbital.
- Molecular orbitals are characterised by a set of quantum numbers.
- Aufbau rule, Pauli’s exclusion principle and Hund’s rule are applicable to molecular orbitals.
- Their shape is governed by the shape of atomic orbitals eg by s – s and p – p overlapping we have
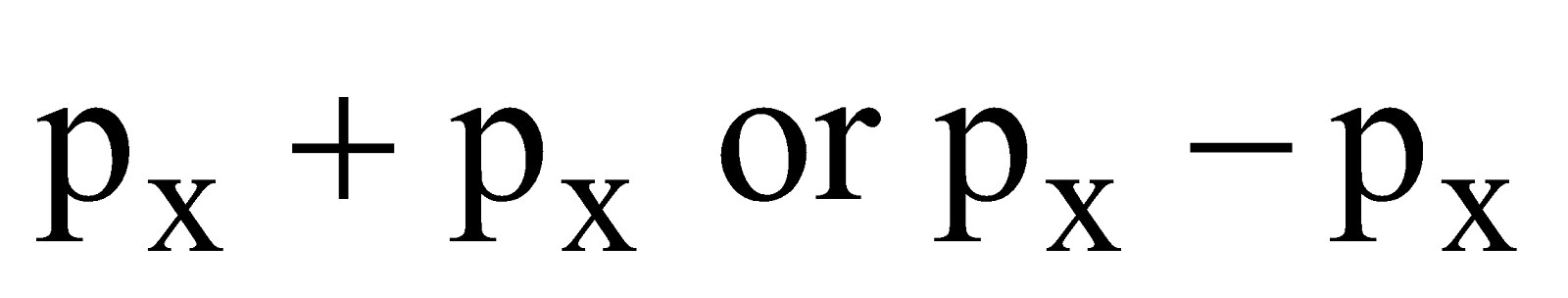 and
and 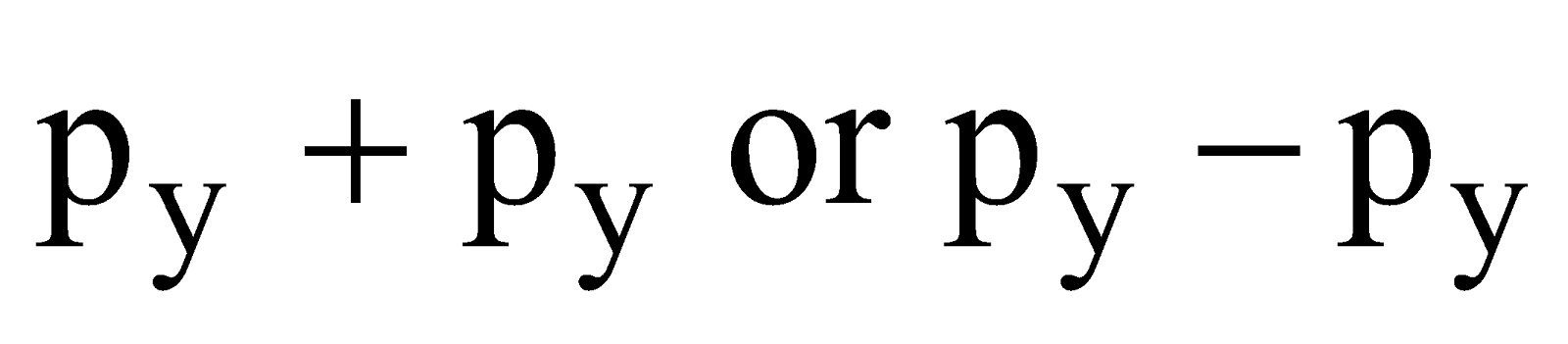 overlap to form bond.
overlap to form bond.
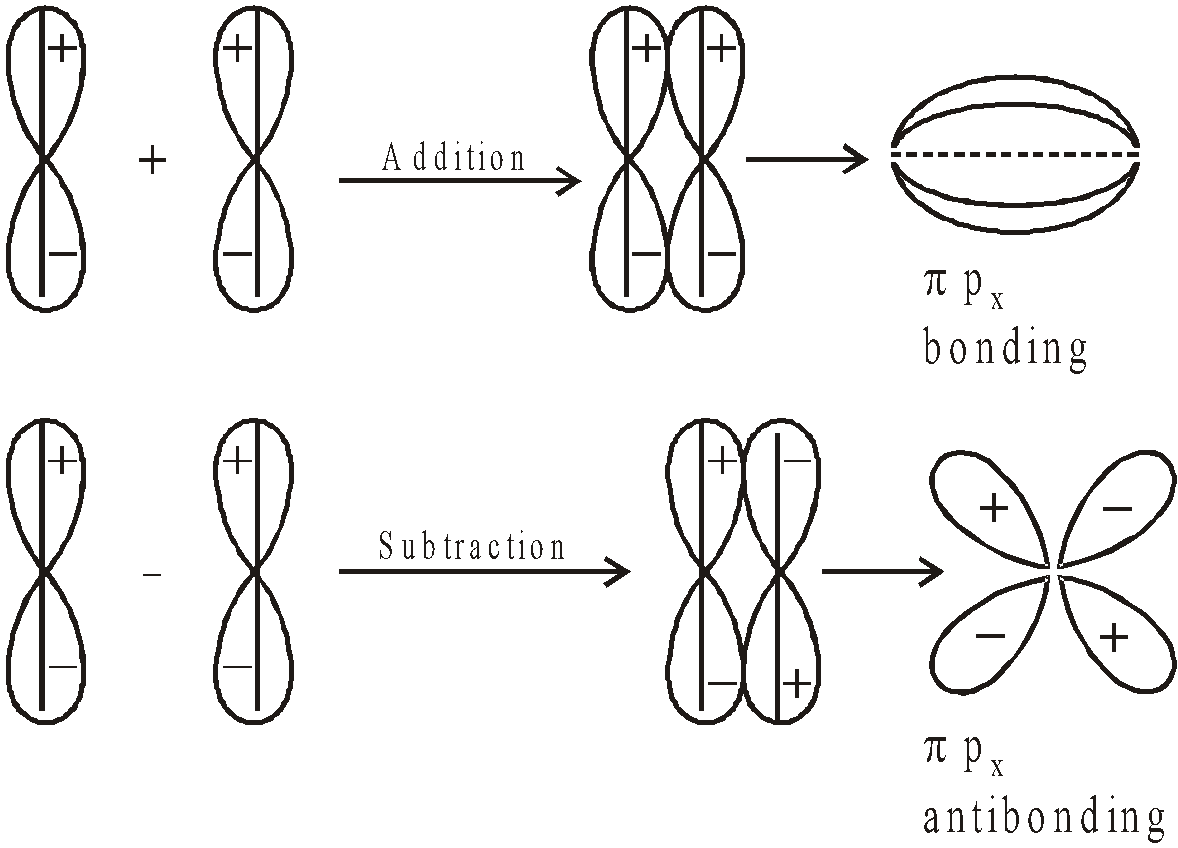
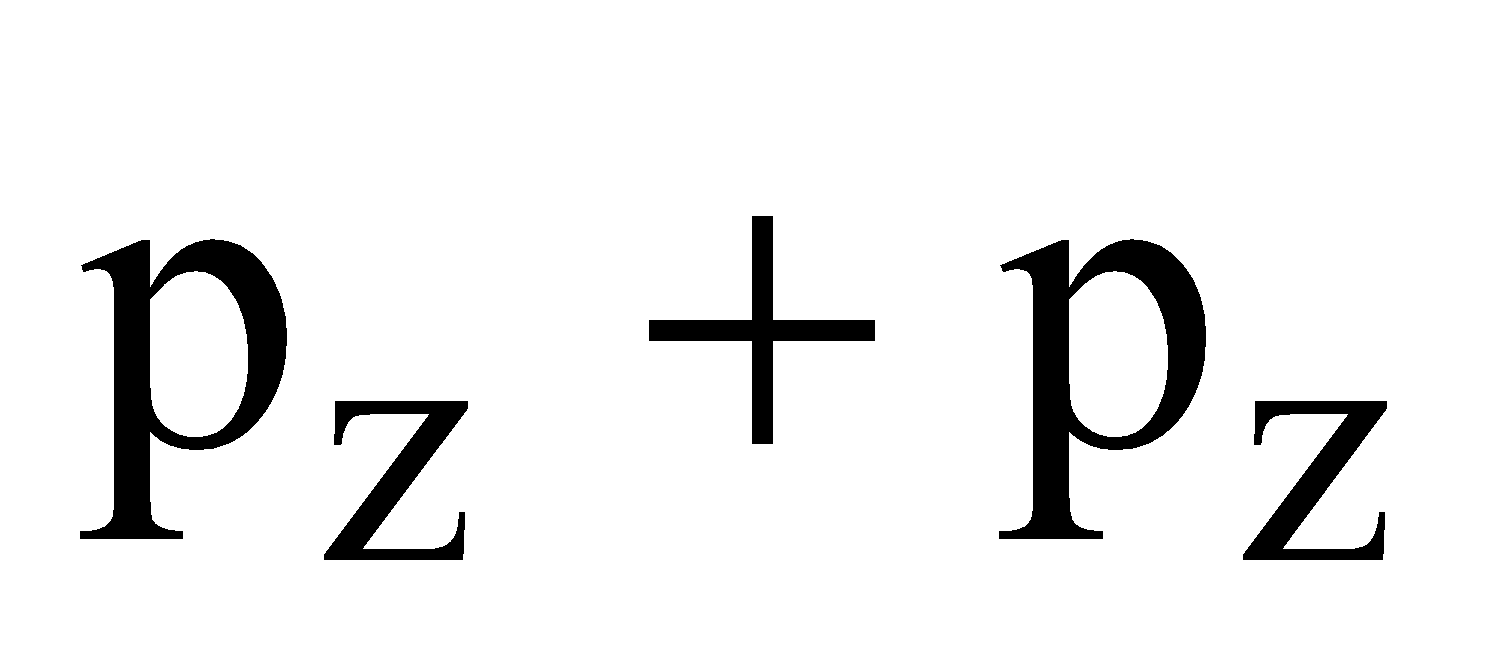 form
form 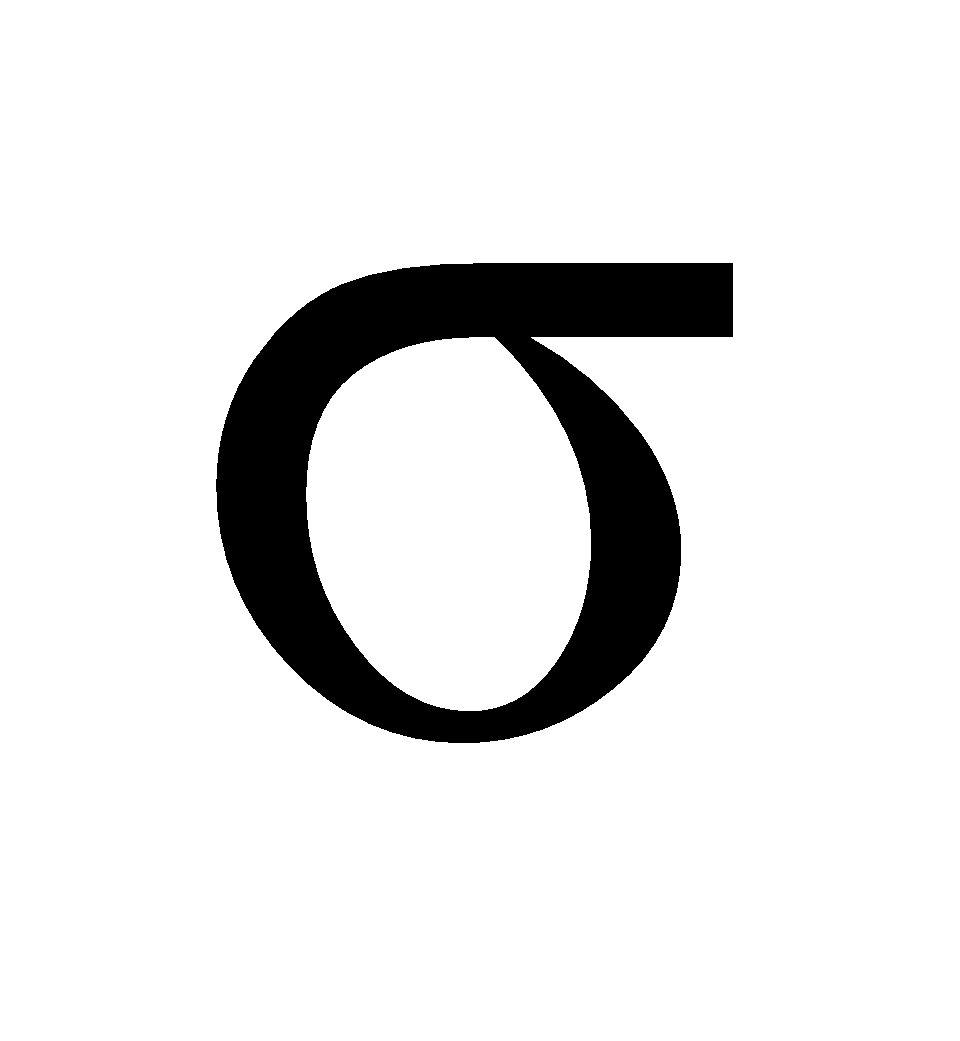 bond and their combination is according to inter-nuclear distance.
bond and their combination is according to inter-nuclear distance.
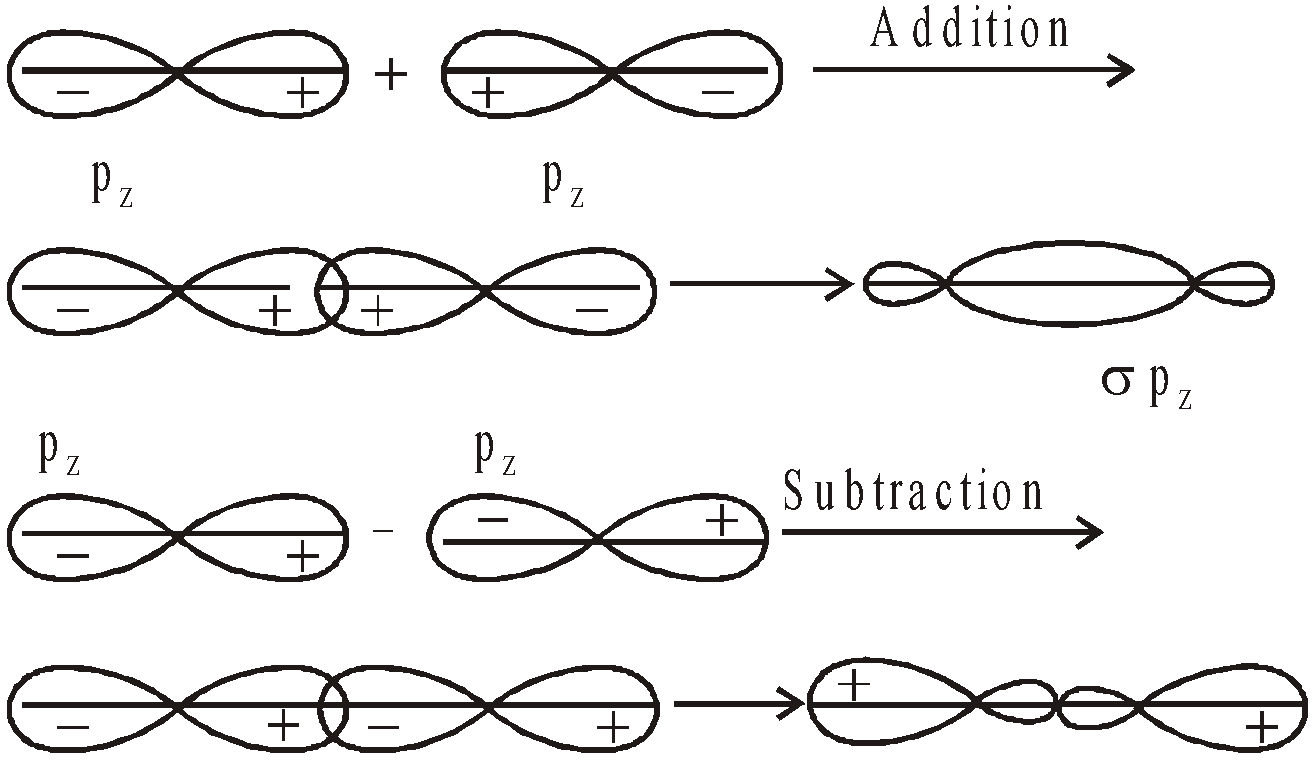
ENERGY LEVEL DIAGRAM
When two atoms A and B are brought nearer to one another, the atomic orbitals present in atom A undergo overlapping with atomic orbitals present in atom B, having the same energy and molecular orbitals are formed. There are two types of energy level diagrams
- for molecules upto N2 (here the energy difference between 2s and 2p orbitals is small)
- for molecules after N2 (here the energy difference between 2s and 2p orbitals is large and they cannot interact)
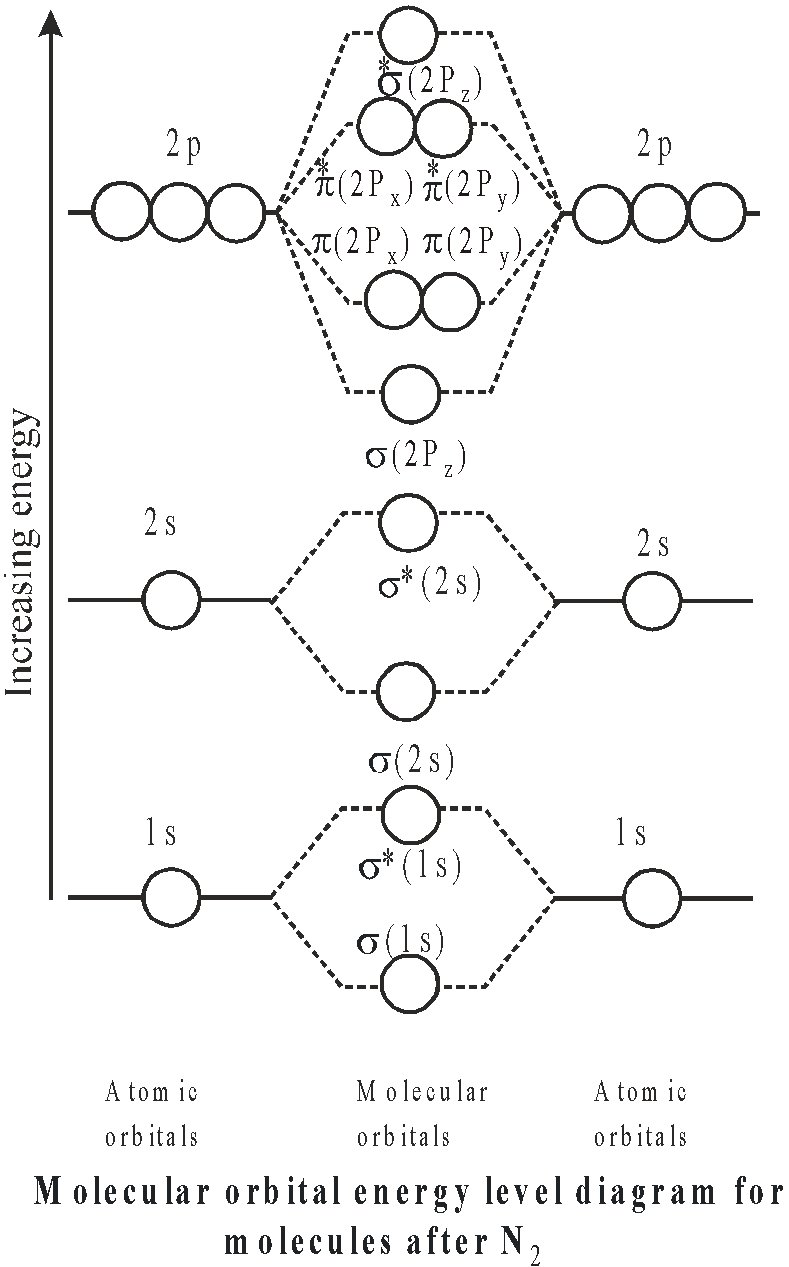
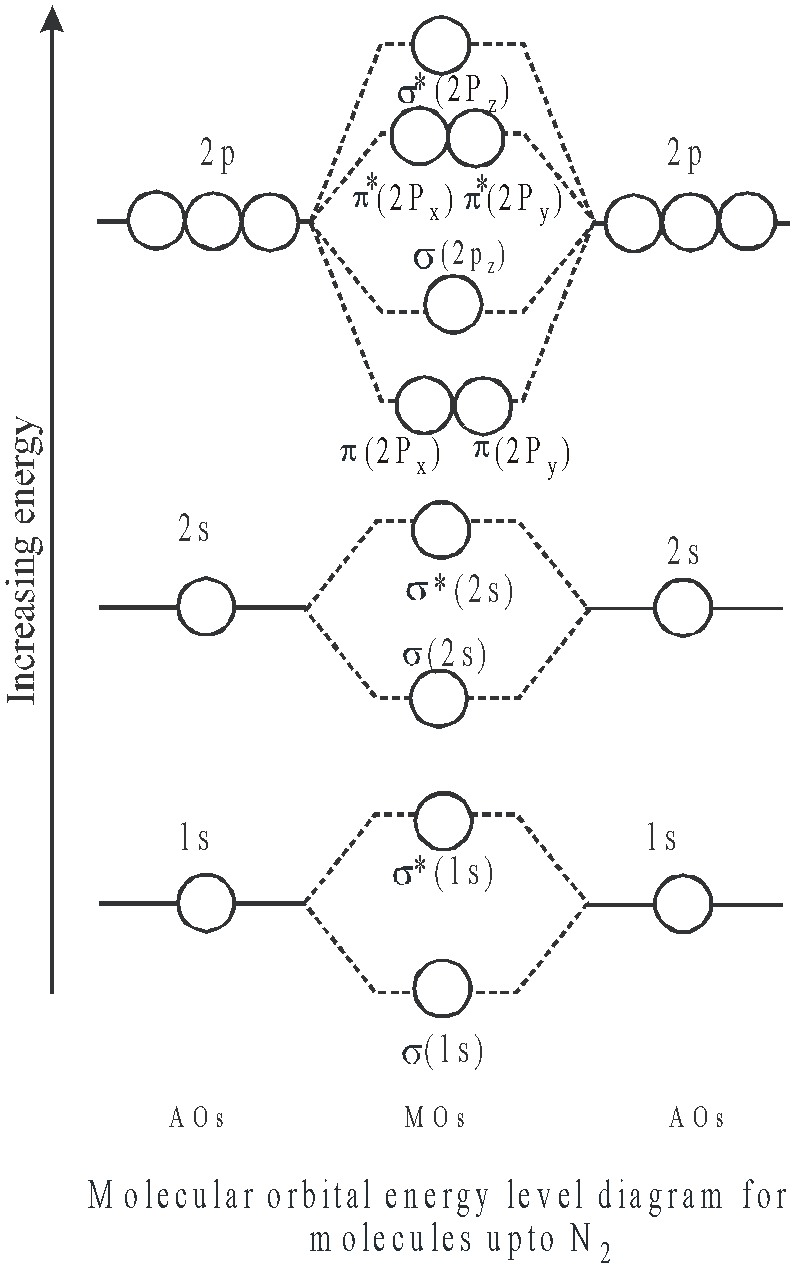
Simply we can write
ELECTRONIC CONFIGURATION / BOND ORDER OF SIMPLE DIATOMIC MOLECULES
The electronic configuration and the bond order in case of simple diatomic molecules can be obtained by filling the molecular orbitals by applying Aufbau principle and Hund’s rule
Bond order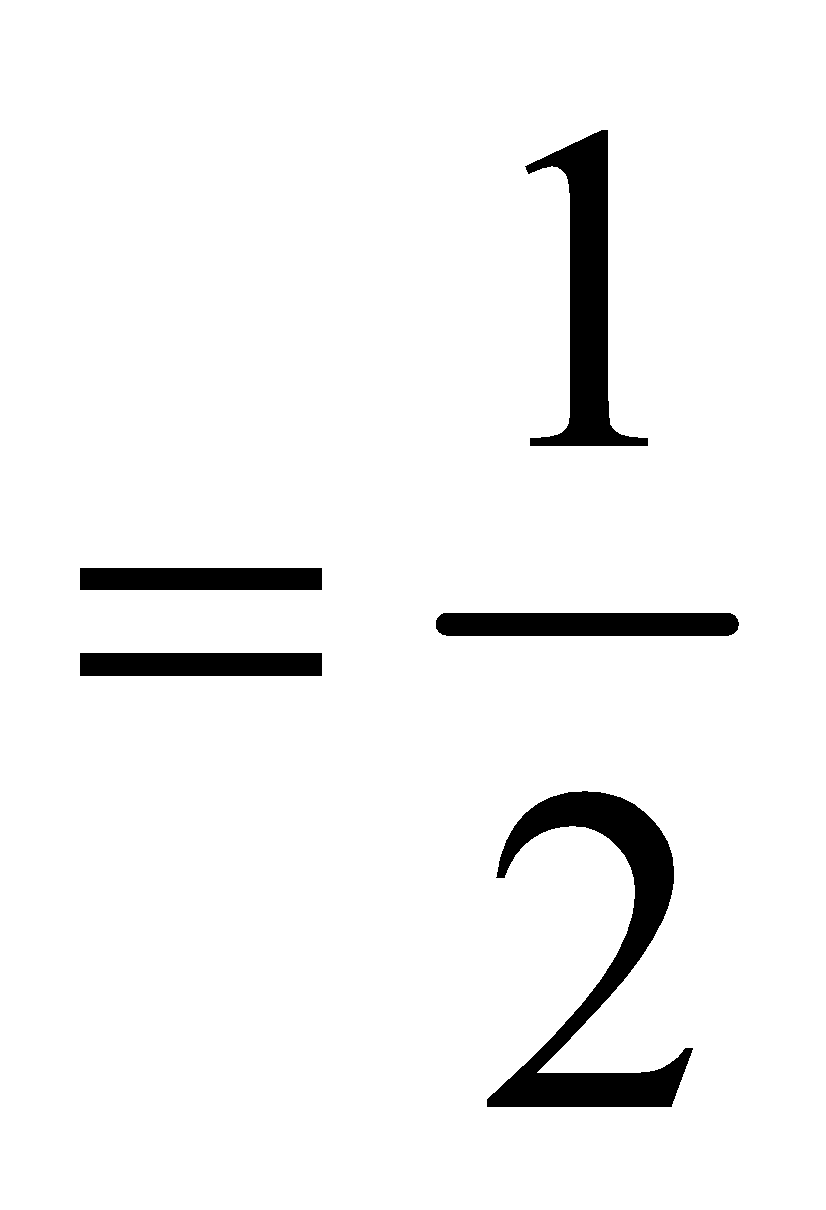 [Number of bonding electrons – Number of antibonding electrons]
[Number of bonding electrons – Number of antibonding electrons]
The magnetic properties of molecules can also be ascertained
BONDING IN SOME DIATOMIC MOLECULES AND IONS
- Hydrogen molecule (H2) – Total number of electrons = 2, filling in molecular orbitals we have

Bond order =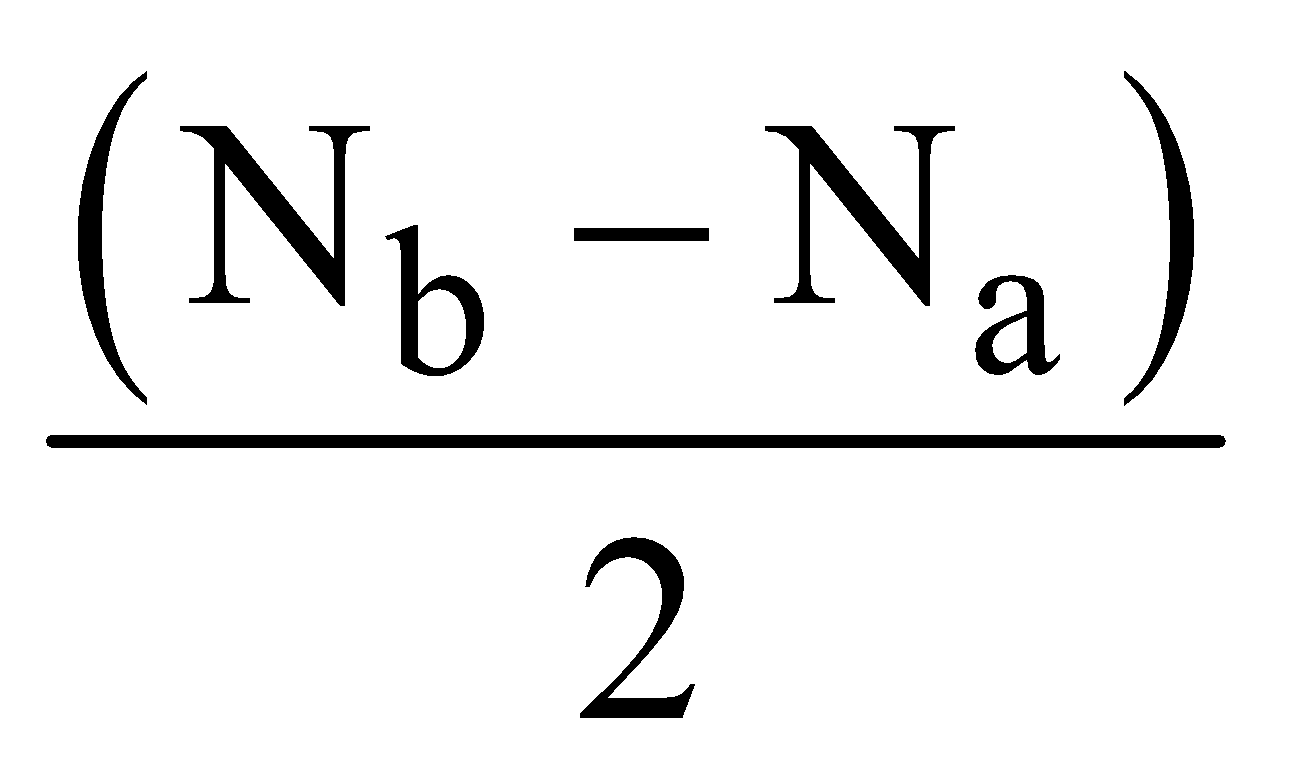
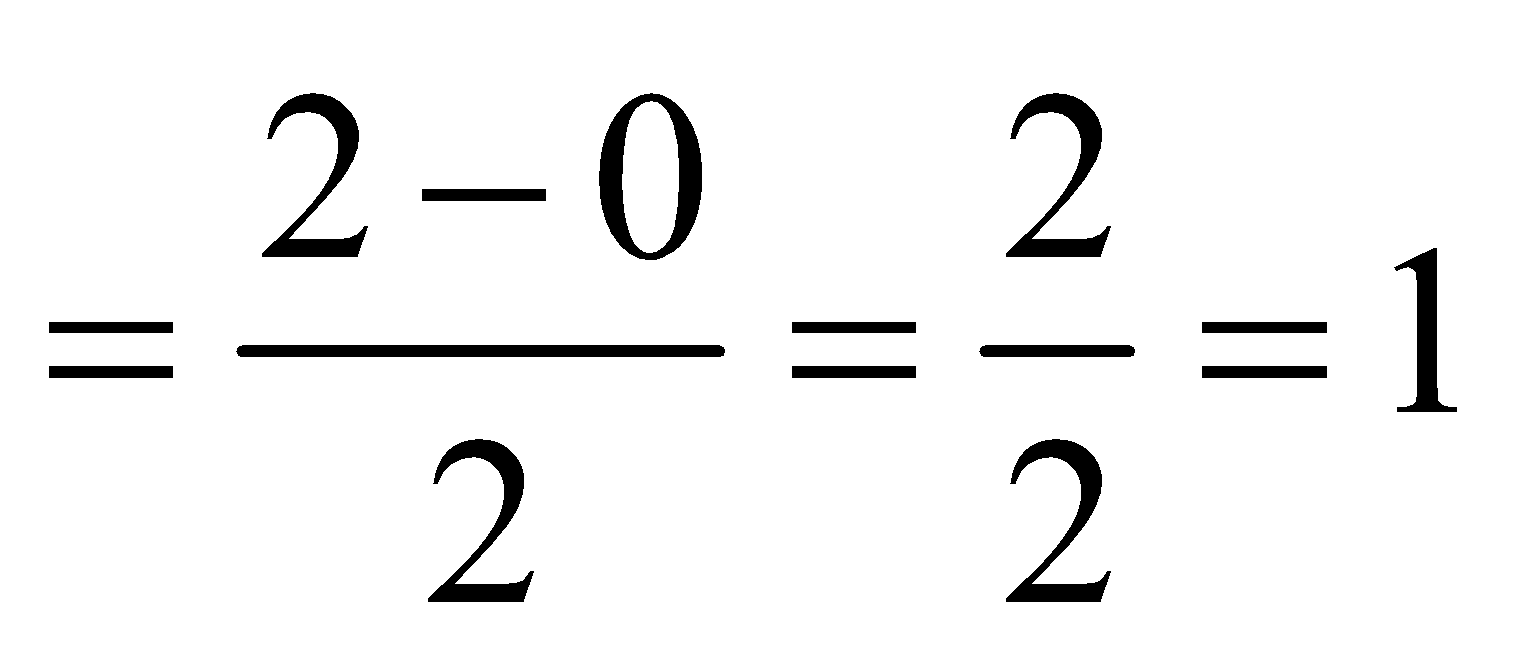
Hence there is a single bond between two hydrogen atoms (H – H). Since there is no unpaired electron, H2 is diamagnetic
- Helium molecule (He2) – The total number of electrons = 4 and filling in molecular orbitals we have

Bond order 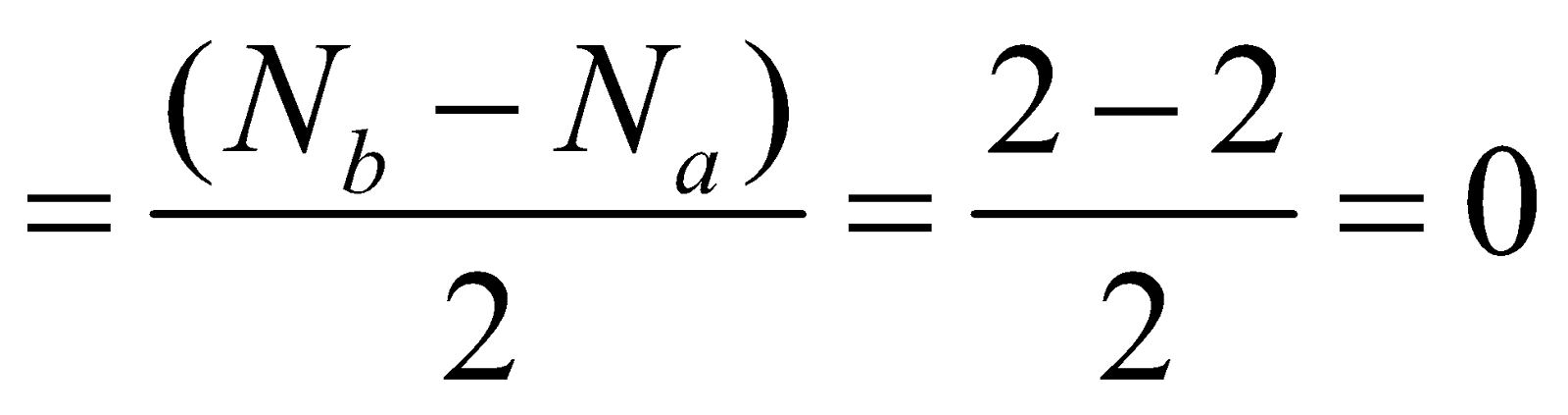
Hence He2 molecule cannot exist
- Nitrogen molecule (N2) – The total number of electrons = 14 and filling in molecular orbitals we have
Bond order 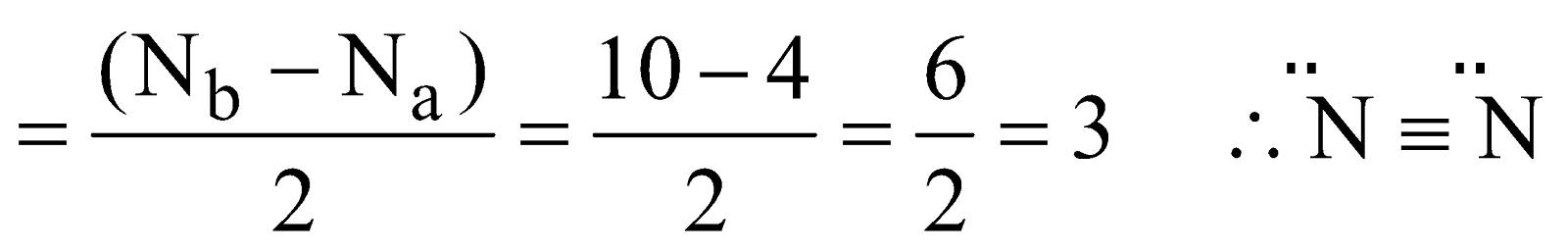
- Oxygen molecule (O2) – Total number of electrons = 16 and electronic configuration is
Bond order = 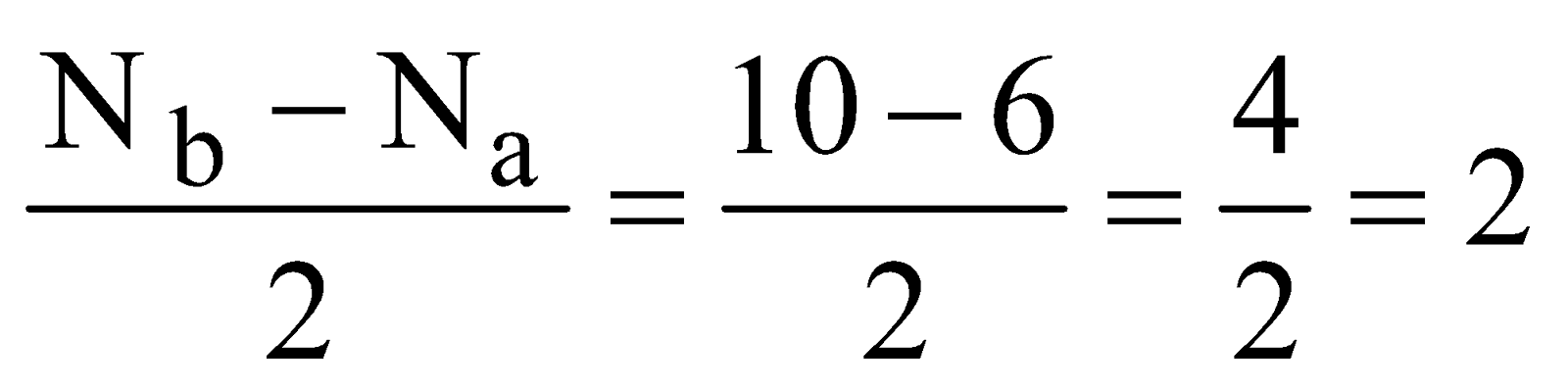
As shown by electronic configuration the O2 molecule contains two unpaired electrons, hence it is paramagnetic in nature
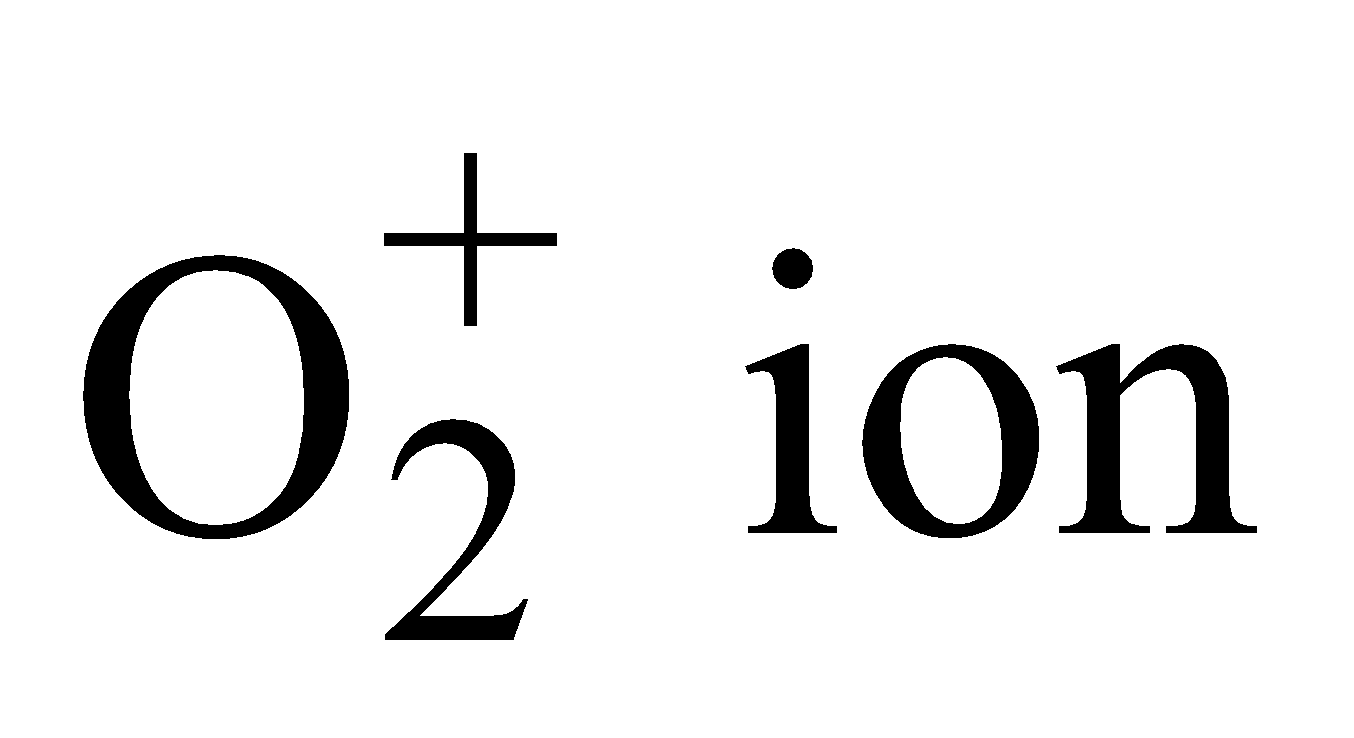 – Total number of electrons (16 – 1) = 15.
– Total number of electrons (16 – 1) = 15.
Electronic configuration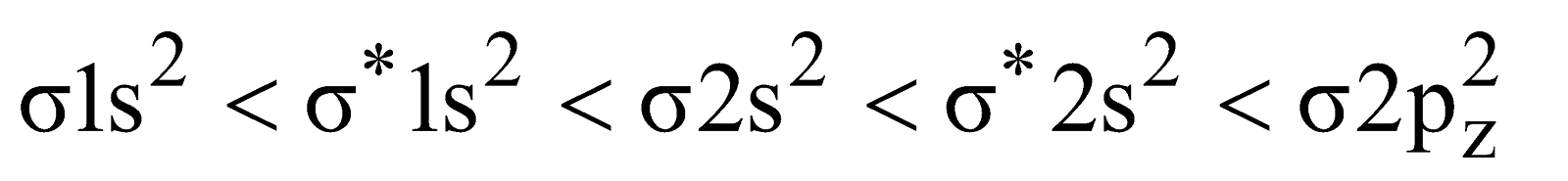
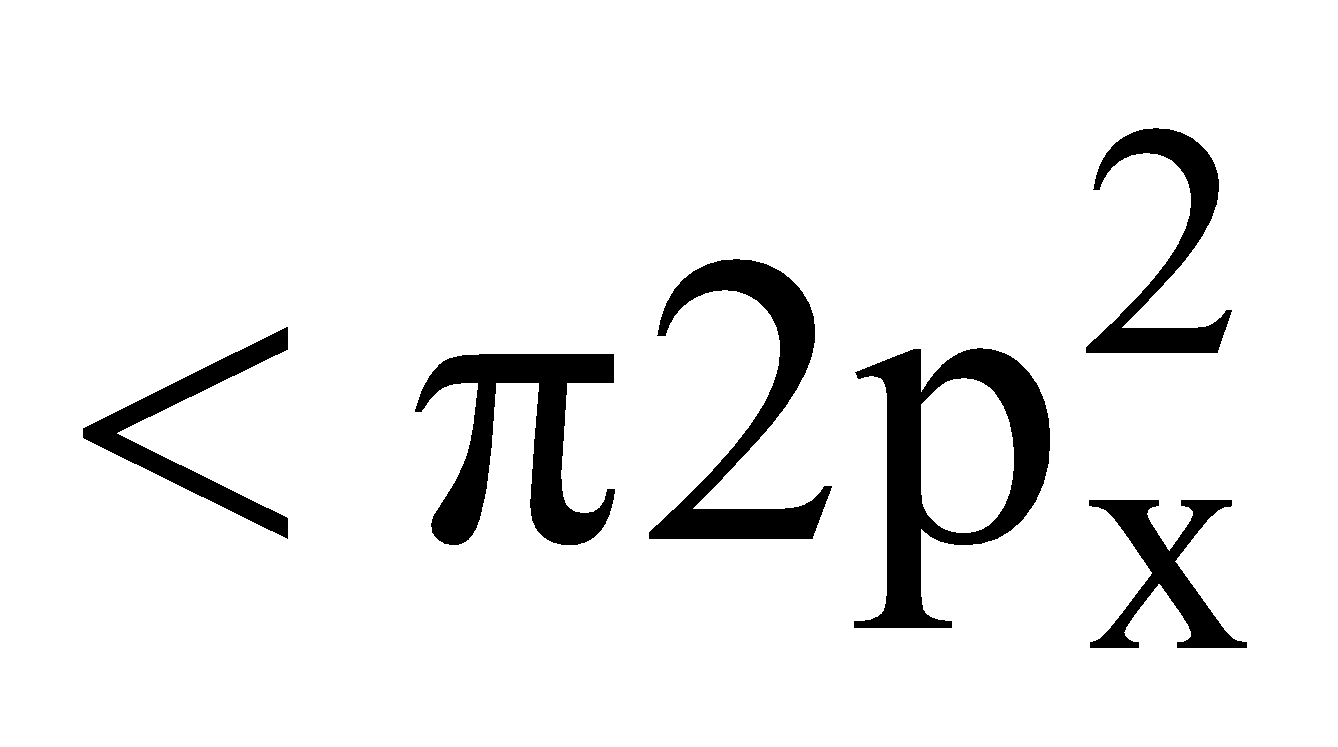
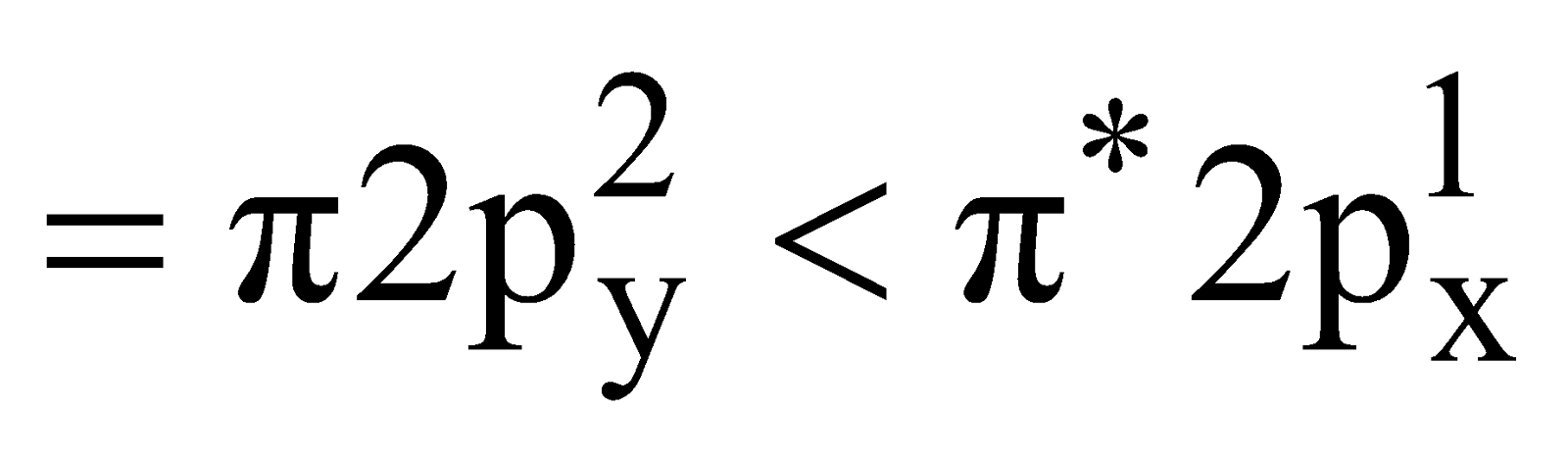
Bond order 
It is paramagnetic
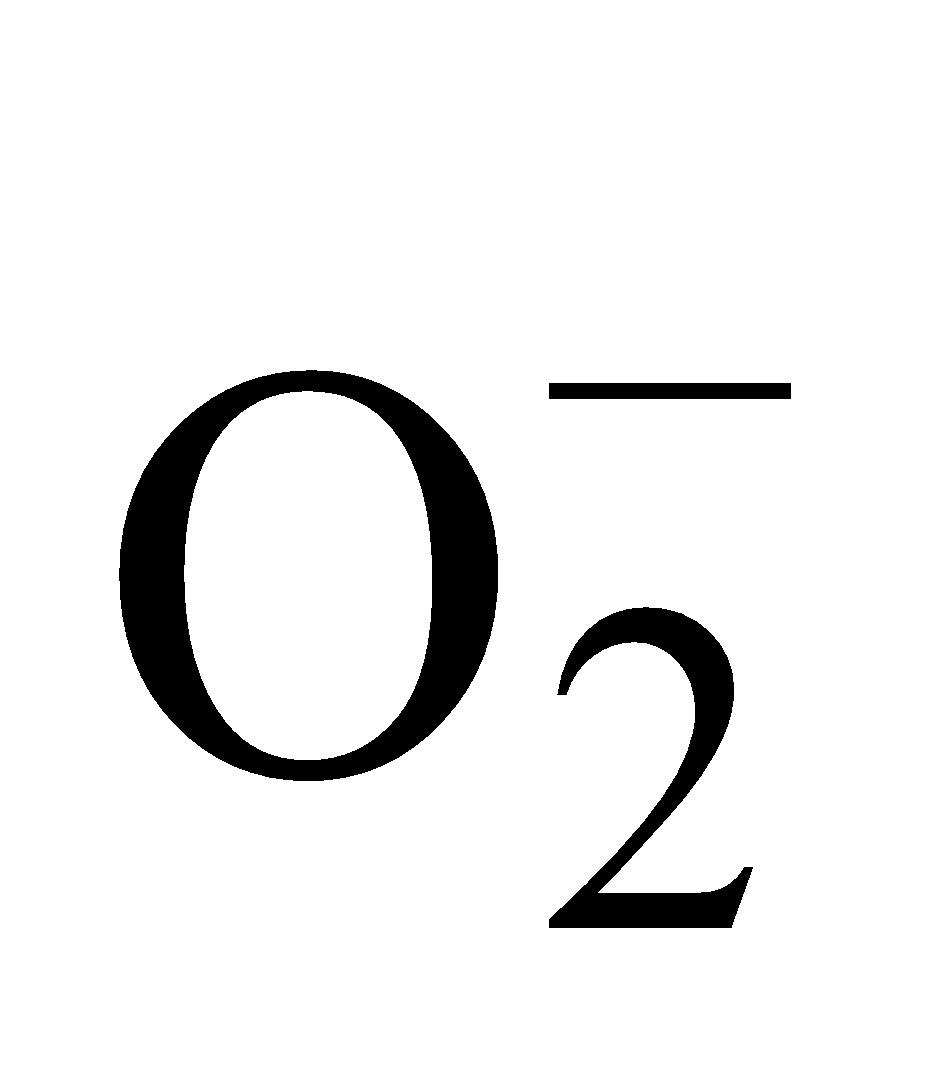 (Super-oxide ion) Total number of electrons
(Super-oxide ion) Total number of electrons  .
.
Electronic configuration
Bond order 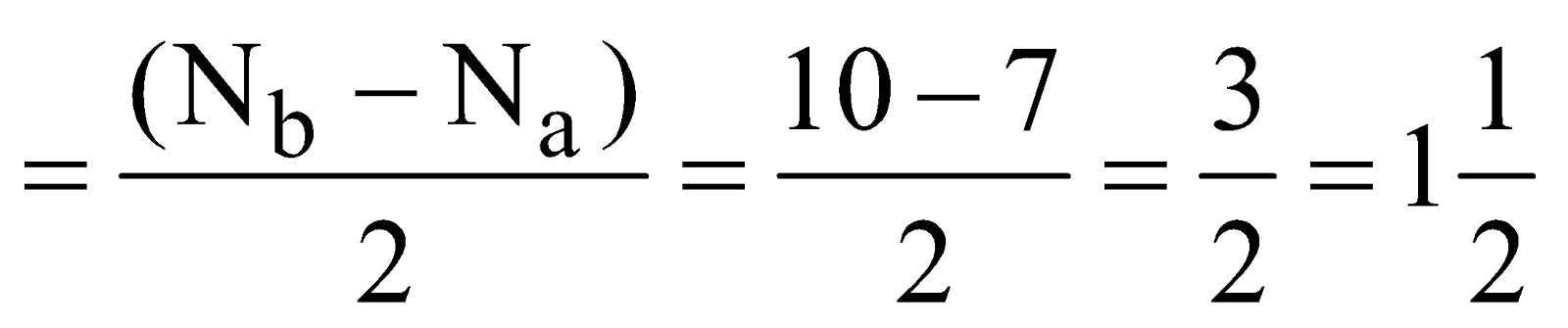
It is paramagnetic
- Peroxide ion –
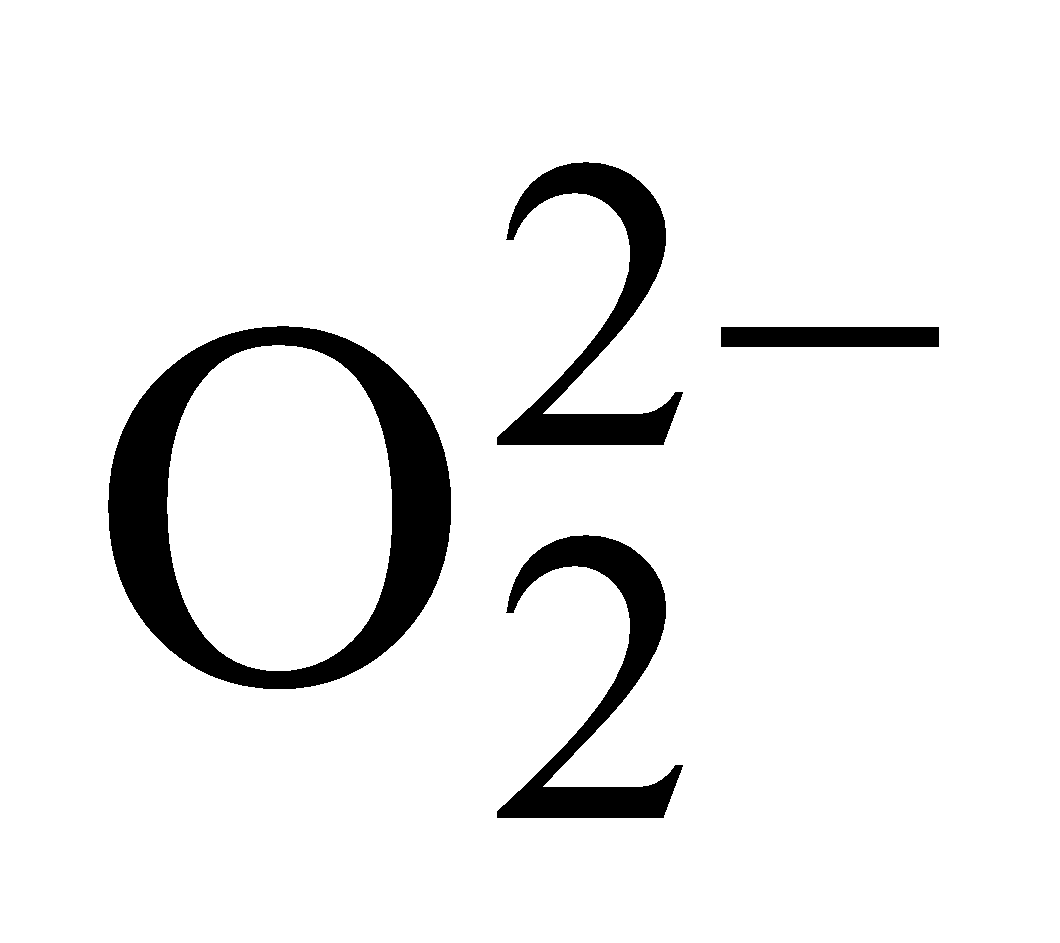 Total number of electrons
Total number of electrons 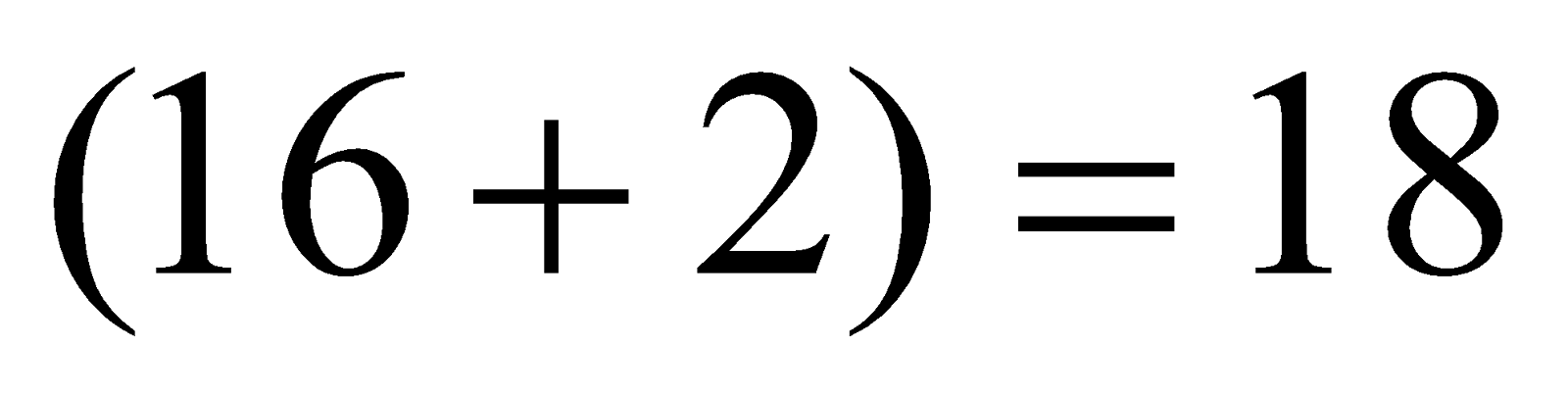 .
.
The electronic configuration is
Bond order 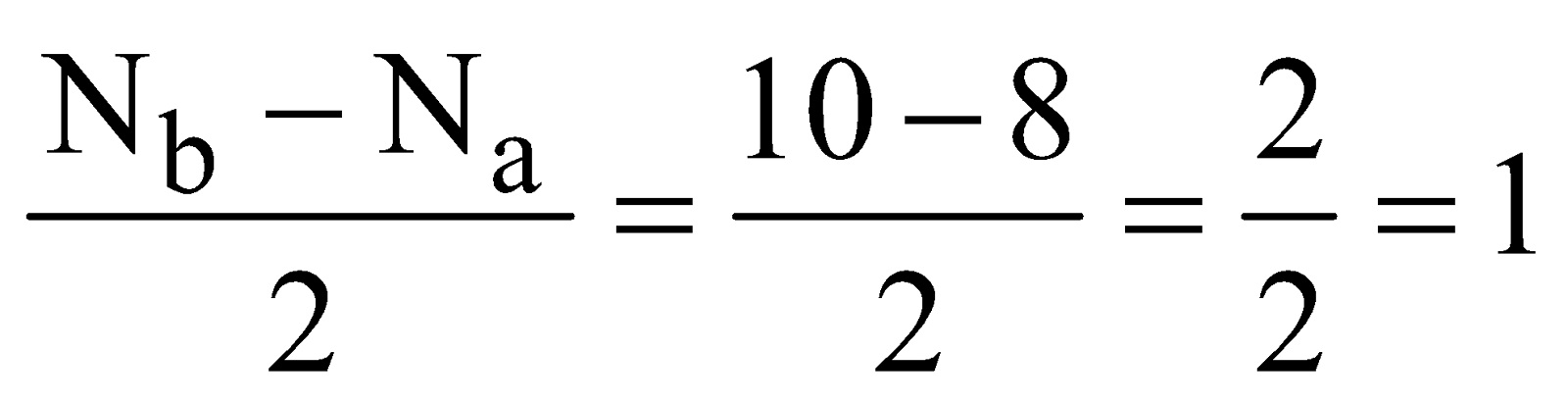
It is diamagnetic
- Carbon monoxide (CO) – Total number of electrons = (6 + 8) = 14
The electronic configuration is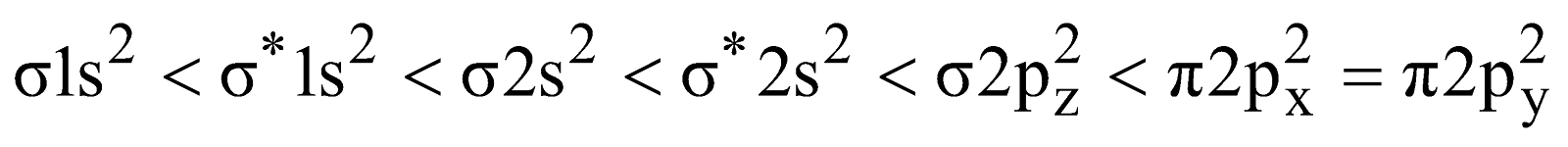
Bond order 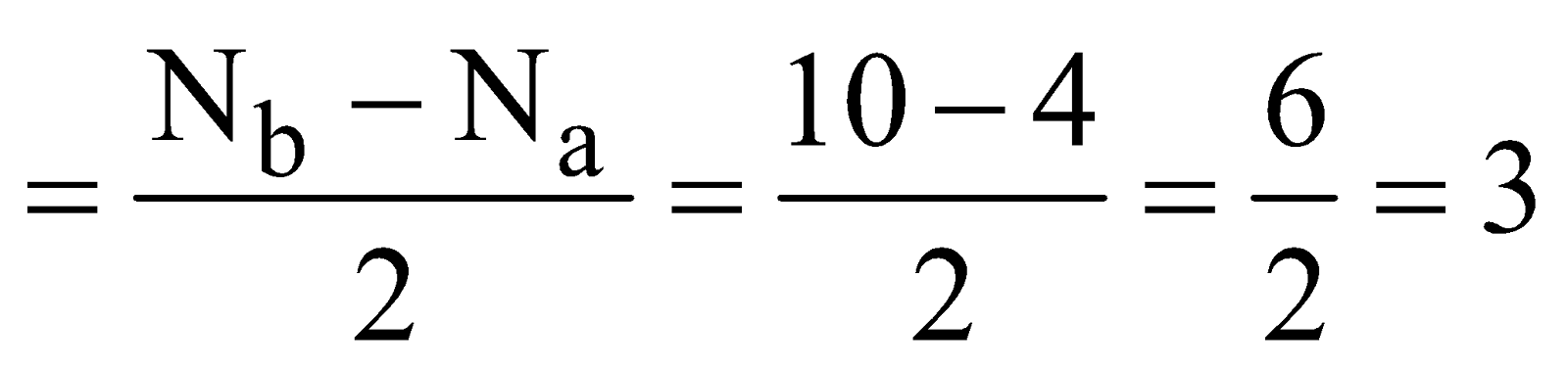
It is diamagnetic
- Nitric Oxide (NO) – Total number of electrons = (7 + 8) = 15
Bond order 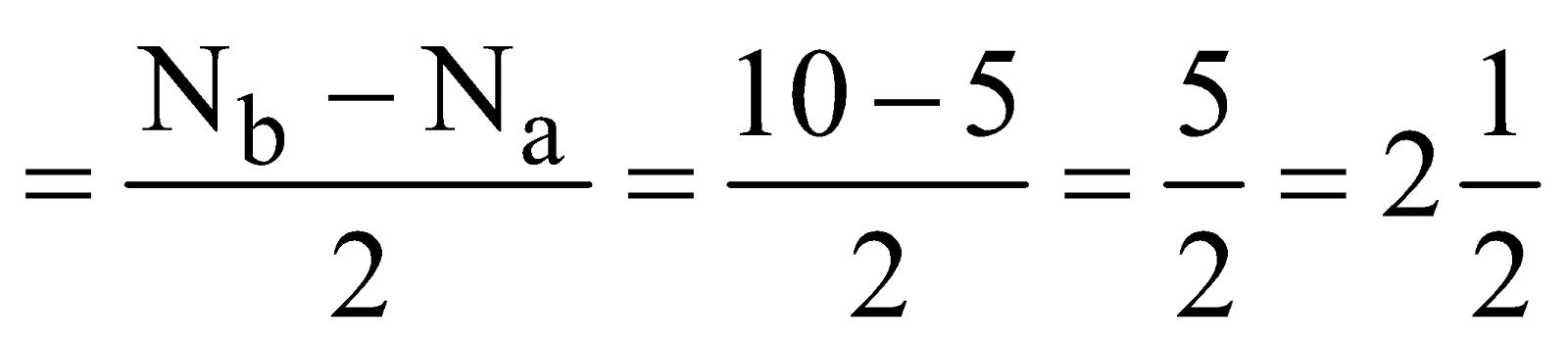
It is paramagnetic.
IONIC CHARACTER OF A COVALENT BOND
- By electronegativity difference – It is determined by the electronegativity difference of the participating atoms. The higher the electronegativity difference between the two atoms, more is the percentage ionic character of a covalent bond. The mathematical equations for calculating, the % ionic character are
- Pauling equation % ionic character
- Hannay and smith equation
= 16 (xA – xB) + 3.5 (xA –xB)2
where xA and xB are the electronegativities of atoms
Percentage ionic character and the electronegativity difference
xA – xB value % ionic character xA – xB value % ionic character
0.1 0.5 1.7 51.0
0.4 4.0 2.0 63.0
0.7 12.0 2.3 74.0
1.0 22.0 2.6 82.0
1.4 39.0 2.9 88.0
- By dipole moment – By dipole moment the % ionic character of a covalent bond can be obtained from the following equation
% ionic character 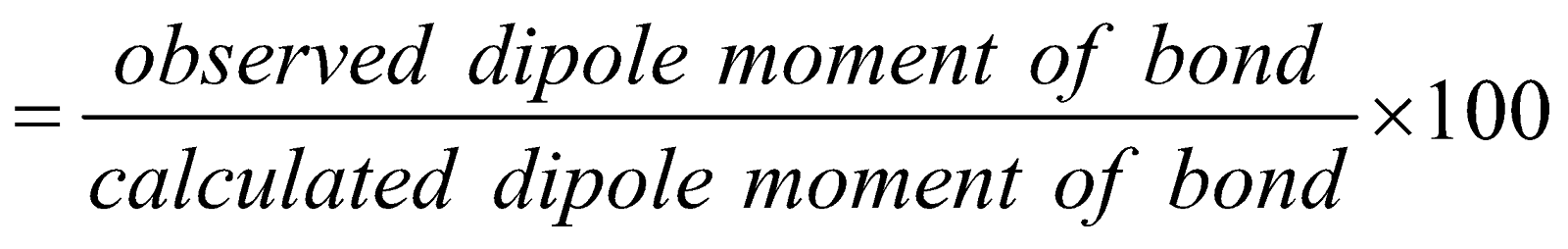
CHARACTERISTICS OF COVALENT COMPOUNDS
- Physical state – They may be gases, liquids or solids
- Crystal structure – They may exist as simple molecules held together by weak forces, giant molecules united by covalent link eg diamond, silicon carbide, alumina, aluminium nitride etc and can have layer structure. eg graphite.
- Solubility – Generally soluble in non polar solvents.
- Melting/ boiling points – Low as compared to ionic compounds.
- Electrical conductivity – Being non electrolytes they do not conduct electricity, graphite does due to presence of free electrons.
- Stereo-isomerism – They exhibit structural and stereo-isomerism both
HYBRIDISATION
Hybridisation is the redistribution of energy levels at the time of formation of molecules. It may also be defined as intermixing of atomic orbitals of nearly the same energy and resulting in the formation of new atomic orbitals same in number and identical in all respects (shape, energy and size). The new atomic orbitals are known as hybrid atomic orbitals.
The completely filled or half filled atomic orbitals can take part in hybridisation and hybrid atomic orbitals form stronger bonds.
METHOD FOR FINDING THE TYPE OF HYBRIDISATION
Apply the following formula to find the hybridisation of central atom.

Value of z 2 3 4 5 6 7
Hybridisation sp sp2 sp3 sp3d sp3d2 sp3d3
Examples :
- Hybridisation of NH3 = [5 + 3 + 0 – 0] = 4; sp3
- Hybridisation of H2O = [6 + 2 + 0 – 0] = 4; sp3
- Hybridisation of SO3 = [6 + 0 + 0 – 0] = 3; sp2
- Hybridisation of SO42– = [6 + 0 + 2 – 0] = 4; sp3
- Hybridisation of CO32– = [4 + 0 + 2 – 0] = 3; sp2
- Hybridisation of PCl5 = [5 +5 + 0 – 0] = 5; sp3d
- Hybridisation of SF6 = [6 + 6 + 0 – 0] = 6; sp3d2
Note : Species having same hybridisation are isostructral in nature.
VSEPR THEORY VALENCE SHELL ELECTRON PAIR REPULSION THEORY
The following types of repulsions between different pair of electrons change the regular geometry of the molecule.
Lone pair – lone pair repulsion > Lone pair – bond pair repulsion > Bond pair – bond pair repulsion or lp – lp > lp – bp > bp – bp eg:
Lone pair – lone pair repulsion > Lone pair – bond pair repulsion > Bond pair – bond pair repulsion or lp – lp > lp – bp > bp – bp eg:
- Shape of H2O molecule – The hybridisation of oxygen is sp3 and angle should be 109º 28’. But actually the HOH angle is 104.5º. The VSEPR theory explains this fact
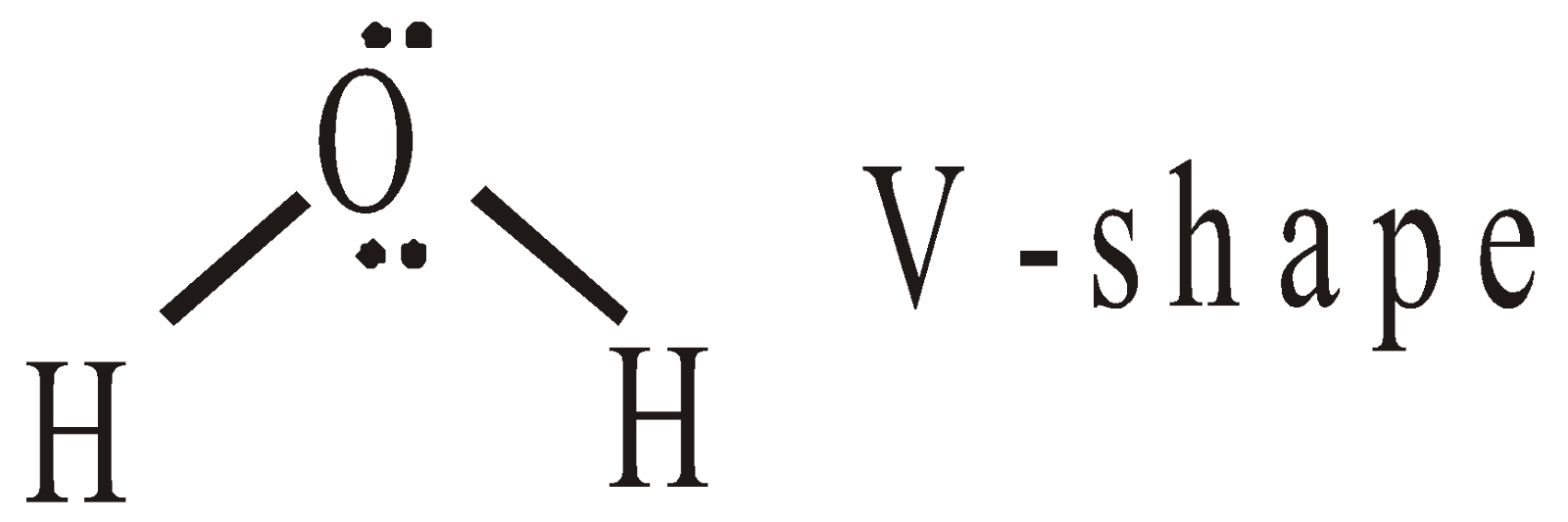
All types of repulsions (lp–lp, lp–bp, bp–bp) are present in H2O.
- Shape of NH3 molecule – The hybridisation of nitrogen is sp3. The H–N–H angle is 107.5º instead of 109º28’. Due to lp–bp and bp–bp repulsions the value of angle change
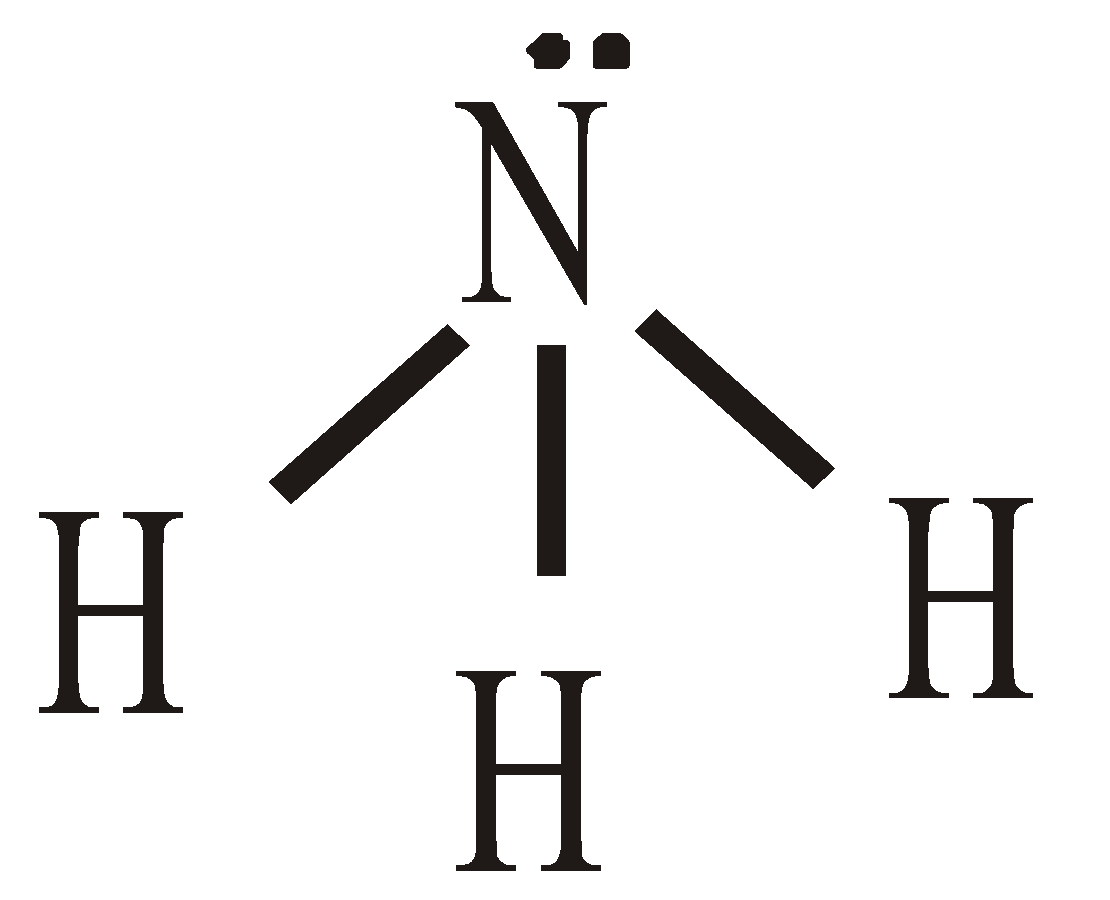 Pyramidal
Pyramidal - Structure of PCl5 – The phosphorus is in sp3d hybridised form. The five hybrid orbitals are not equivalent. The bonds pointing towards the three corners of equilateral triangle are known as equatorial bonds making an angle of 120° between them. The remaining two bonds are at right angle to the plane of three make an angle of 90° and are called axial bonds. The axial bonds are slightly longer and weaker than equatorial bonds. Since they suffer more repulsive interaction from the later. Consequently PCl5 is very reactive molecule.
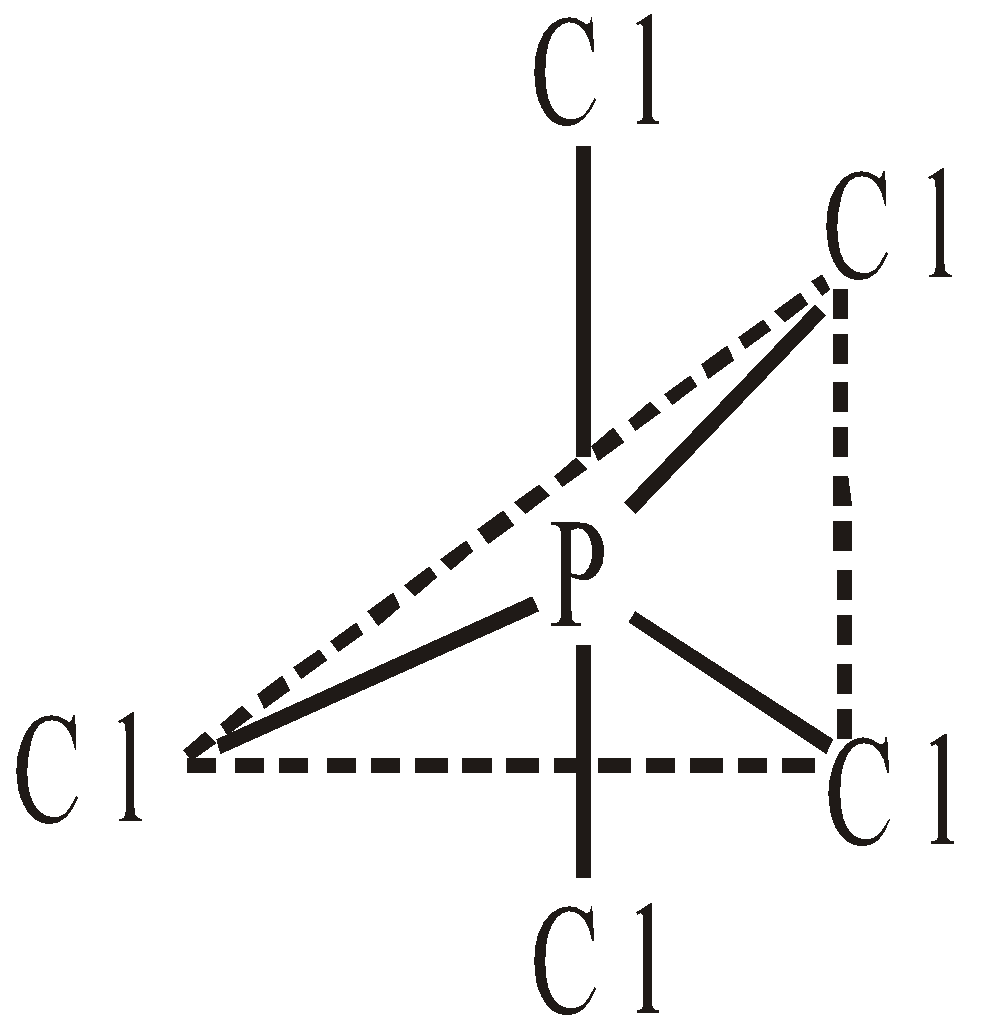
COORDINATE COVALENT BOND OR DATIVE BOND
When both the electrons for sharing between two atoms are contributed by one atom only the bond formed is known as coordinate bond or dative bond.
In terms of orbital theory the coordinate covalent bond is formed by overlapping between empty and completely filled atomic orbitals.
The atom donating the pair of electrons is called donor and the atom which accepts the pair of electrons is called acceptor. The compounds containing coordinate bonds are known as coordination compounds. The bond is represented by an arrow ( ) pointing head towards the acceptor.
) pointing head towards the acceptor.
Once the coordinate bond is formed it is indistinguishable from a covalent bond. Examples
- Formation of SO2
- Formation of SO3
- Formation of Hydroxonium ion
- NH3 and BF3 form addition product by Coordinate covalent bond
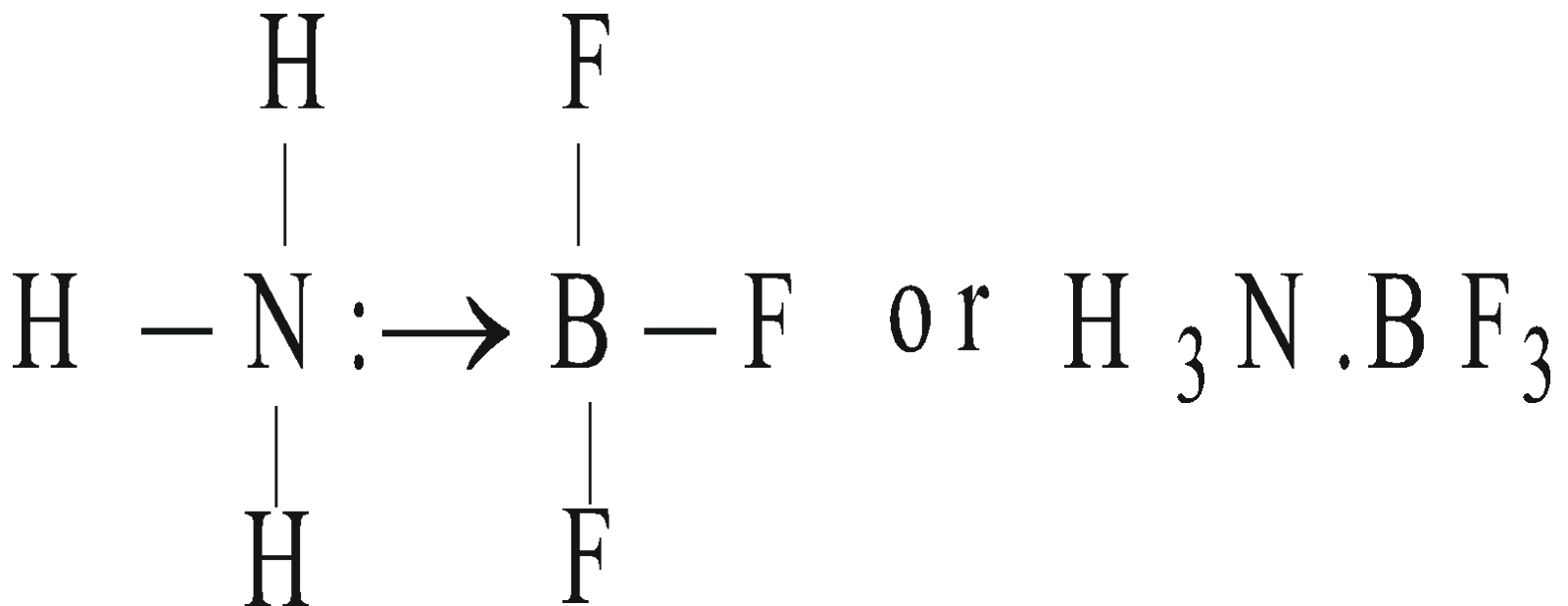
CHARACTERISTICS OF COORDINATE COMPOUNDS
Coordinate compounds have volatile character in between ionic and covalent compounds. Other properties like solubility, electrical conductivity and stereo-isomerism are similar to covalent compounds.
HYDROGEN BOND
It may be defined as the force of attraction existing between hydrogen atom covalently bonded to highly electronegative atom (N, O or F) and the electronegative atom belonging to another molecule of the same or different substance. It is represented by dotted lines
H–F ………… H–F ………… H–F
The chains possess a Zig-Zag structure
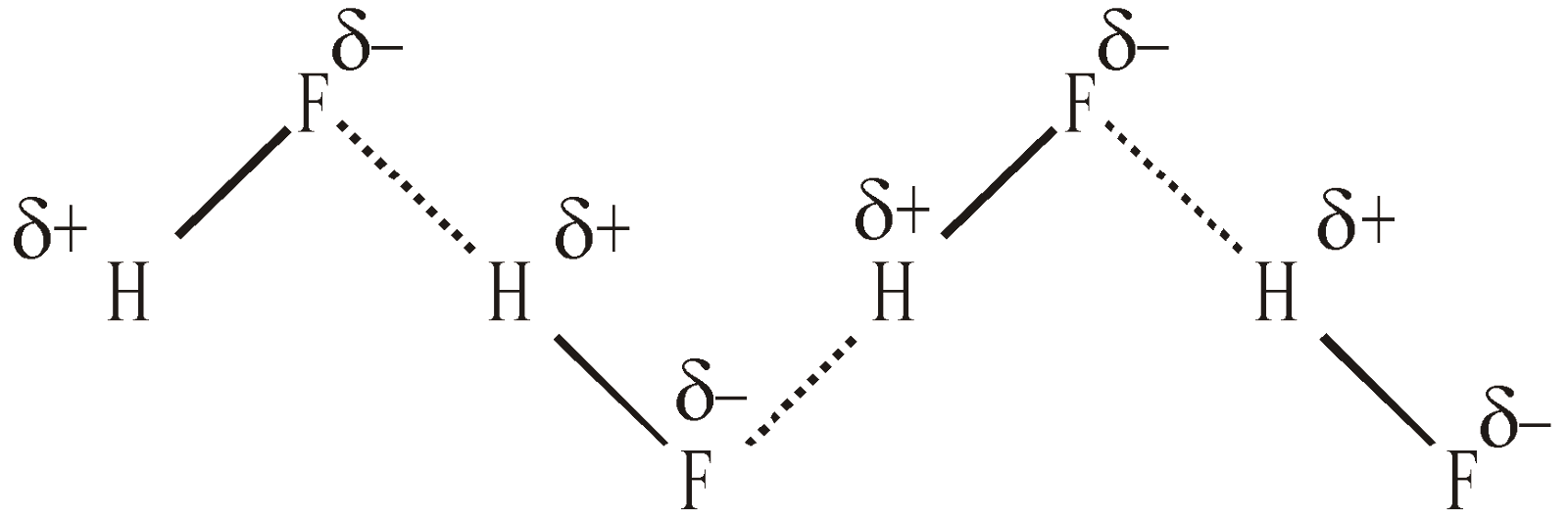
Hydrogen bond is purely electrostatic in nature. It is a weak bond, the strength of the strongest being about 5 – 10 kcal per mole. The more the electronegativity of atom involved in H – bonding, the more is the bond strength eg.
10 kcal/mole > 7 kcal/mole > 2.0 kcal/mole
TYPES OF HYDROGEN BONDS
Hydrogen bond is of two types
- Intermolecular H-bonding (Association). H–bonding involving two or more molecules.
- Intramolecular H-bonding (Chelation). H–bonding taking place within single molecule.
APPLICATIONS OF INTERMOLECULAR H-BONDING
- Water – Water has the lowest molecular weight among the hydrides of group 16 elements yet it has the highest melting and boiling points
H2O H2S H2Se H2Te
Melting point 0ºC -85.5ºC -66ºC -51.2ºC
Boiling point 100ºC -60.4ºC -41.5ºC +2ºC
It is due to intermolecular H – bonding through which water molecules associate
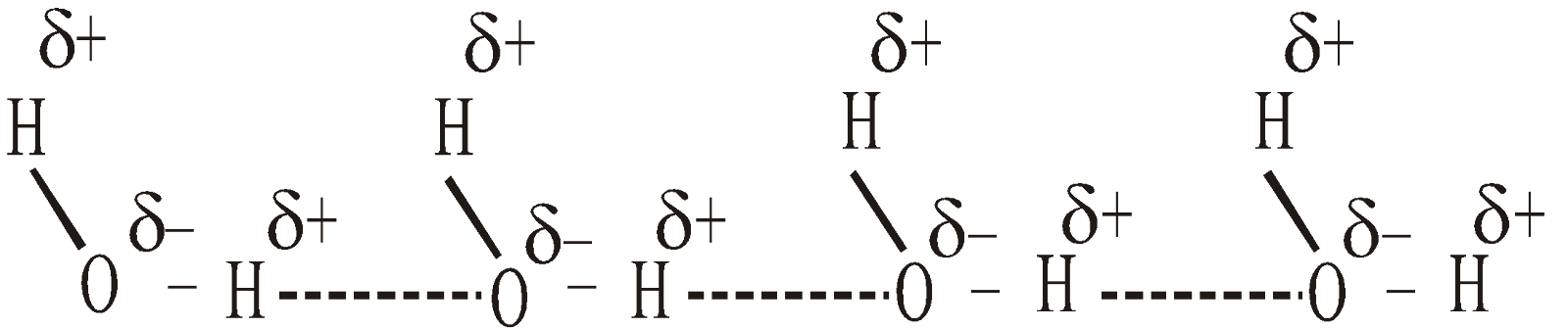
- Ice has less density than water – In crystal structure of ice every water molecule is associated with four other water molecules by H-bonding in a tetrahedral fashion
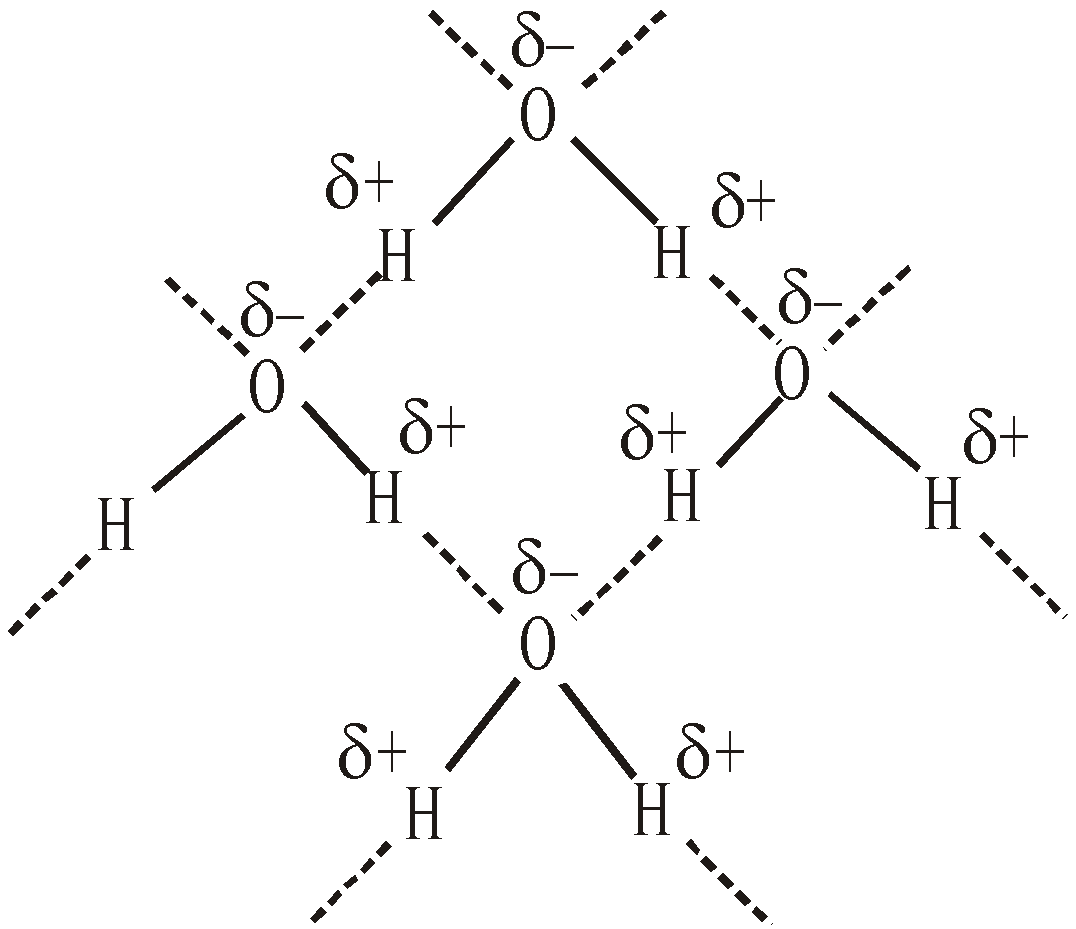
It gives rise to cage likes tetrahedral structure of ice with large empty spaces. On melting the ice H-bonds are broken and space between water molecules decreases and density of water increases upto 4ºC. Above 4ºC more H – bonds are broken, the water molecules move apart from each other and the density again decreases. Thus water has maximum density at 4ºC.
- Alcohols – The marked difference between the melting and boiling points of alcohols and corresponding mercaptans is also due to association.
CH3OH C2H5OH C3H7OH C4H9OH
Boiling point 64.4ºC 78ºC 97ºC 117ºC
CH3SH C2H5SH C3H7SH C4H9SH
Boiling point 5.8ºC 37ºC 67ºC 97ºC
- Amides – Amides associate and have higher melting and boiling points
- Amines – Primary and secondary amines are associated and have higher boiling points than isomeric tertiary amines.
- Fatty acids – In organic solvents, fatty acids form a dimer. It is confirmed by electron diffraction method.
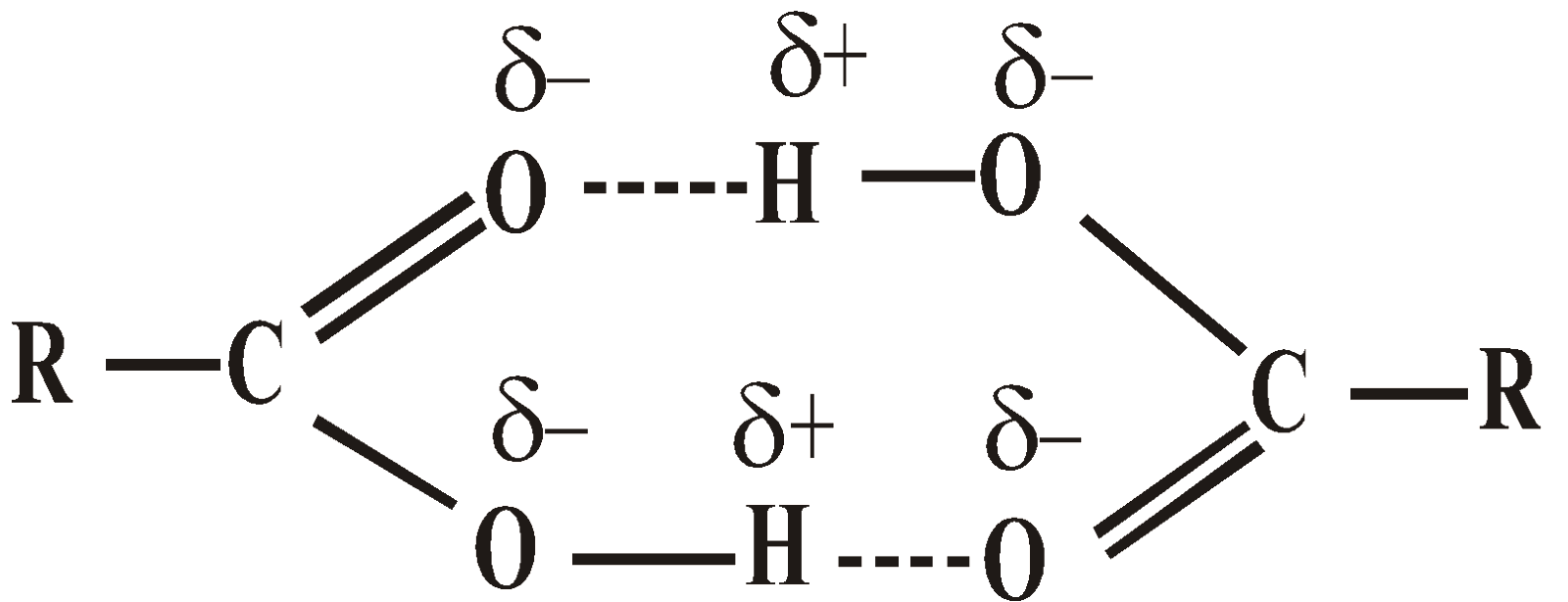
APPLICATIONS OF INTRAMOLECULAR H – BONDING
When the H – bonding takes place within a single molecule this is known as intramolecular H – bonding. It also affects the physical and chemical properties of compound.
- Volatile character of nitrophenols – o-nitrophenol is more volatile (b.pt 214ºC) as compared to meta (b.pt 290ºC) and para (b.pt 279ºC). It is due to chelation
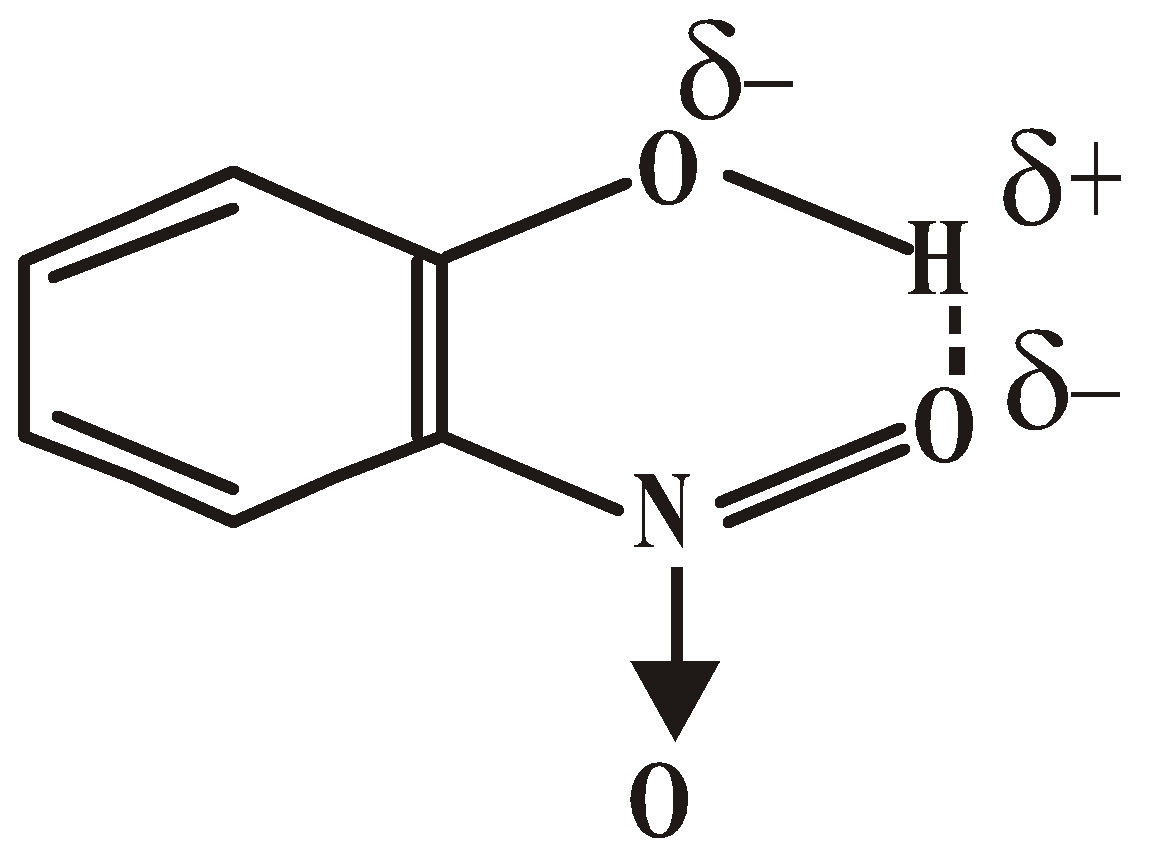
In meta and para isomer chelation is not possible due to the formation of desired size of ring. They therefore associate to some extent and have higher B.P
- Salicylic acid is stronger acid than o – methoxy benzoic acid
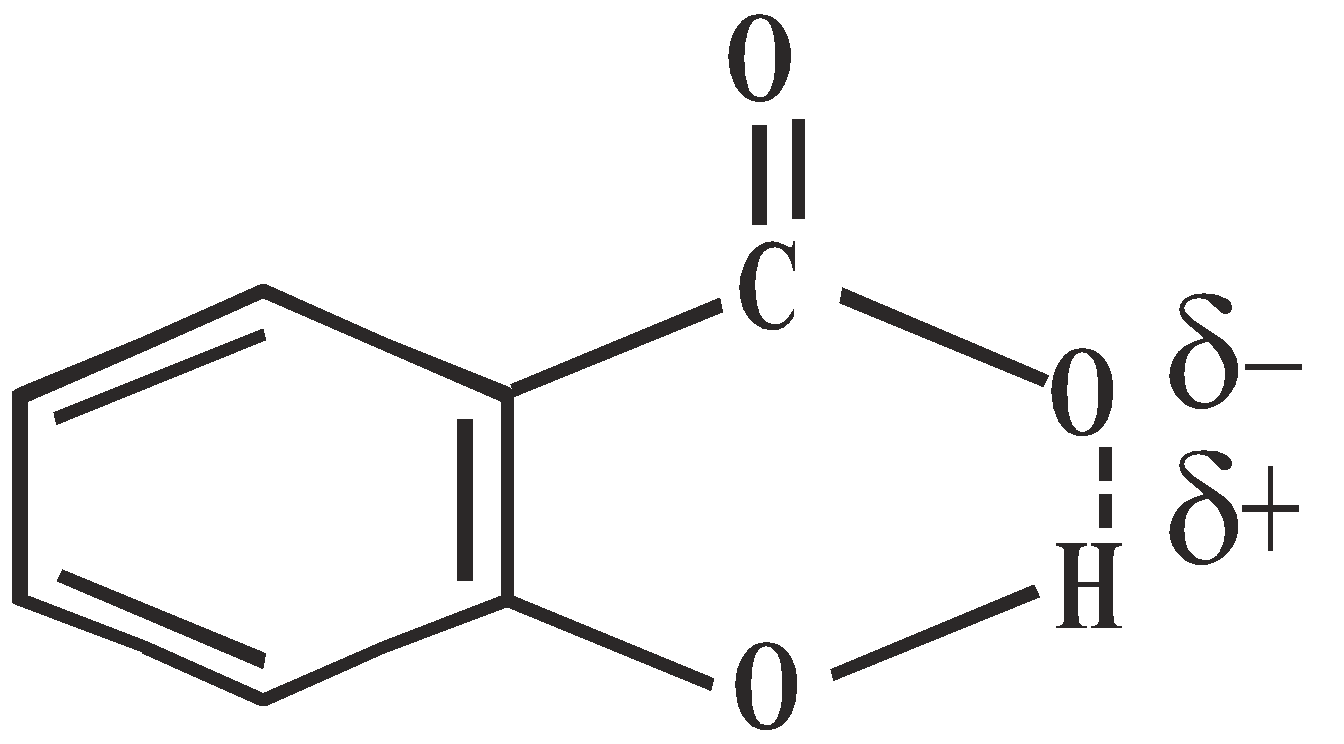
H-bonding in salicylate ion No-H-bonding in o-methoxy benzoate ion
- Ethyl aceto acetate – It exists in two forms
Enolic form is more volatile due to chelation
- Maleic acid is stronger acid than fumaric acid
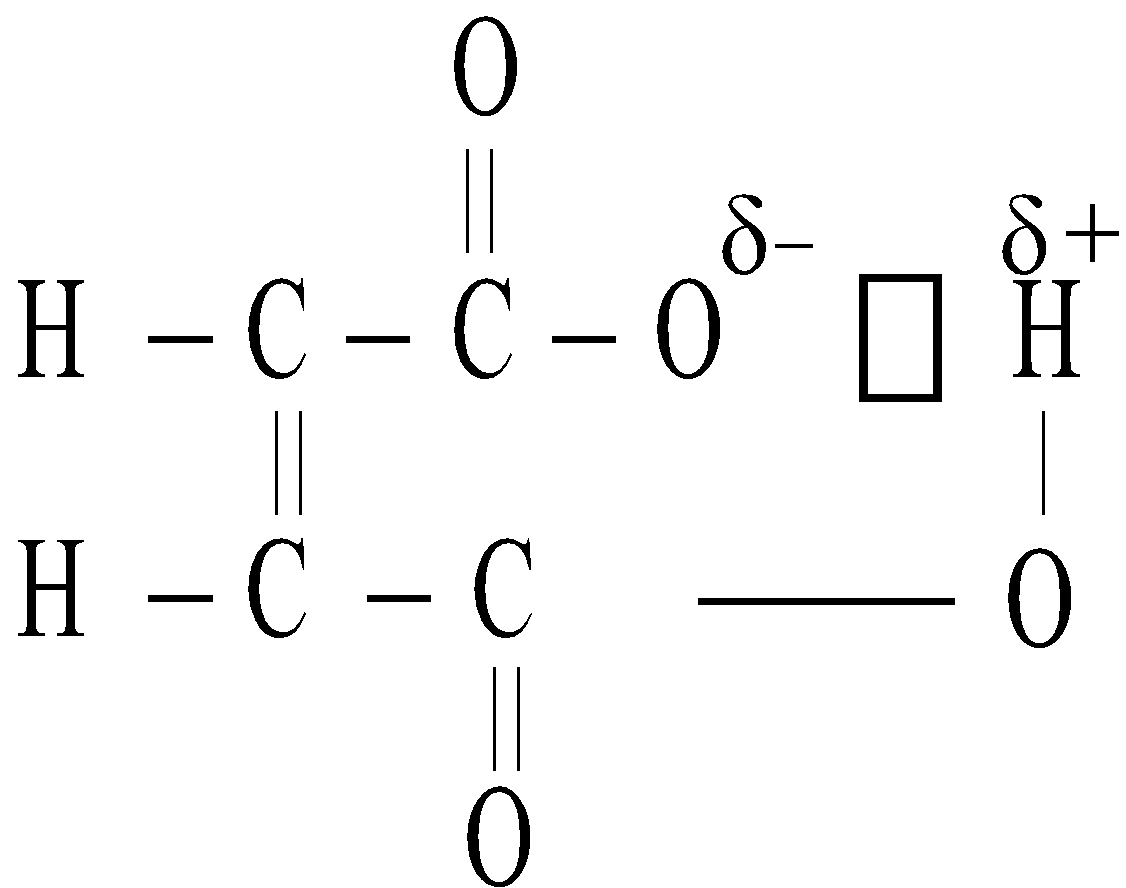
Maleic acid Chelation in maleate ion
- Acid character of nitrophenols. It follows the following order
p – nitrophenol > o – nitrophenol > m – nitrophenol
Acid character of o – nitrophenol is suppressed by chelation
- Other compounds showing intramolecular H – bonding
o-Chlorophenol o-hydroxy benzaldehyde
o-hydroxy benzoic acid
METALLIC BOND
It has been found that metals have generally low ionisation energies indicating that valence electrons are weakly bound to the atomic kernel (or core).
Metals contain few valence electrons and valence orbitals which are empty. Thus valence electrons are completely delocalised and are frequently exchanged between atoms. The atoms thus acquire a positive charge and are arranged in a regular fashion (lattice structure).
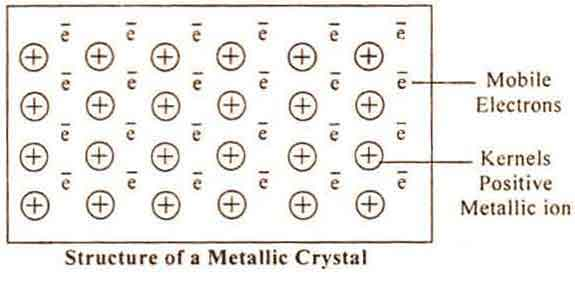
The valence electrons form an “electron gas” or “sea of electrons”. The attractive interaction between the mobile electrons and a number of ions constitutes a weak bond known as metallic bond.
CONSEQUENCES OF METALLIC BOND
- Electrical conductivity – It is due to presence of mobile valence electrons.
- Thermal conductivity – On heating one part of metal, the K.E. of electrons is increased and they conduct heat to the other parts of the metal.
- Metallic lustre – The mobile electrons are promoted to excited states by absorption of light and on coming back from the excited state light of all wavelengths in the visible region is emitted. The surface, therefore emits metallic lustre.
- Malleability – Metals can be made thin sheets
- Ductility – Metals can be drawn into wires since metal kernels can easily be shifted.
- Electrical conductivity decreases with temperature – It is due to random motion of mobile electrons which increases with increase of temperature.
VAN DER WAAL’S FORCES
Attractive forces between uncharged molecules are known as van der Waals forces. These forces may be divided into three groups
- Dispersion or London forces. These are due to transient polarisation.
- Dipole-dipole attraction. These are due to permanent polarisation.
- Dipole-induced dipole forces
LONDON FORCES
The negative electrons in a neutral molecule are balanced by the positive charges on the nucleus. Since the electrons are in motion the centre of density of the electrons does not coincide continuously with the centre of density of positively charged nuclei, the molecules acquire an electric dipole. Polarised molecules exert an attraction for other molecules having a dipole. Consider the case of helium.
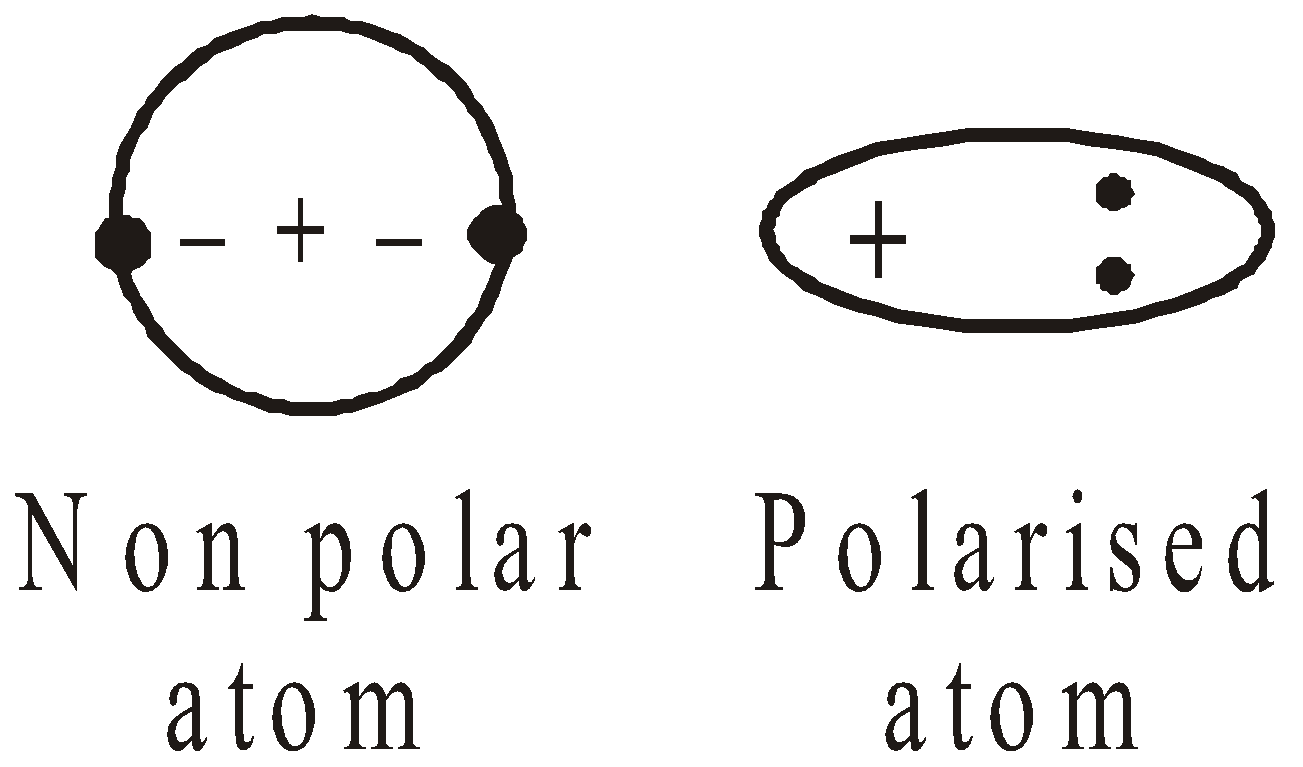

- The greater the polarizability of a molecule, the stronger are the London forces.
- The polarizability increases with number of electrons and their distance from the nucleus.
- These forces are operative over a very short range.
- The inert gases have weak van der Waals forces of attraction
- The straight chain hydrocarbons boil at higher temperature than isomeric branched chain hydrocarbons due to greater magnitude of van der Waal’s forces of attraction.
DIPOLE-DIPOLE ATTRACTION
It is the attraction between the positive end of the one molecule and negative end of the another molecule
This type of interaction is called dipole – dipole interaction. The force of dipole – dipole attraction is inversely proportional to fourth power of their separation of distance r. 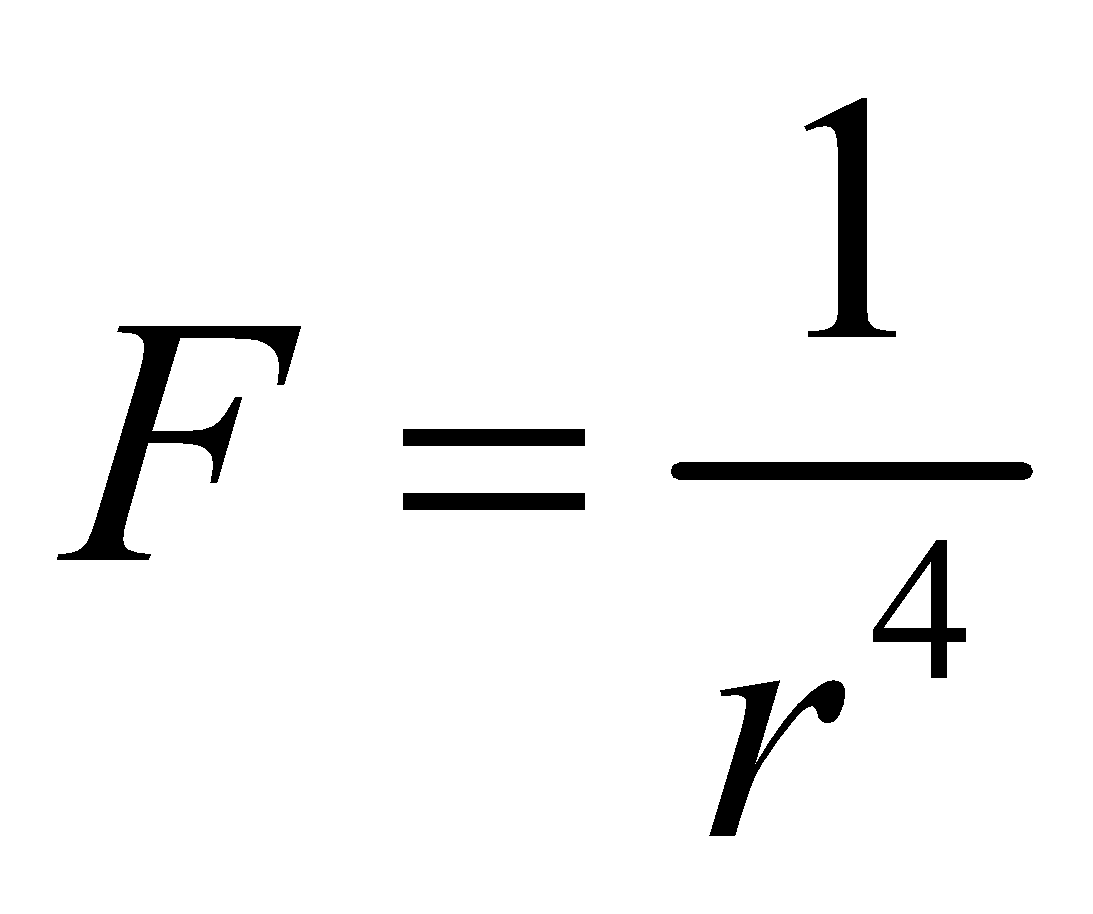 . The dipole dipole attractions in gaseous molecules are particularly small since the gas molecules are in continuous motion. This attraction increases the m.pt. and b.pt. of substances and makes their liquefaction easy.
. The dipole dipole attractions in gaseous molecules are particularly small since the gas molecules are in continuous motion. This attraction increases the m.pt. and b.pt. of substances and makes their liquefaction easy.
DIPOLE-INDUCED DIPOLE ATTRACTION
The attractive forces operate between polar molecules having permanent dipole and non-polar molecules. The polarity in the non-polar molecules is induced by the polar molecule

DIPOLE MOMENT
If a covalent bond is formed between two dissimilar atoms, eg. A and B, one of the atoms (A or B) must be more electronegative than the other. If A is more electronegative than the shared pair of electrons is drawn near A leaving a positive charge on B and hence making the molecule dipolar (A–B+). The percentage of polar character is given in terms of dipole moment (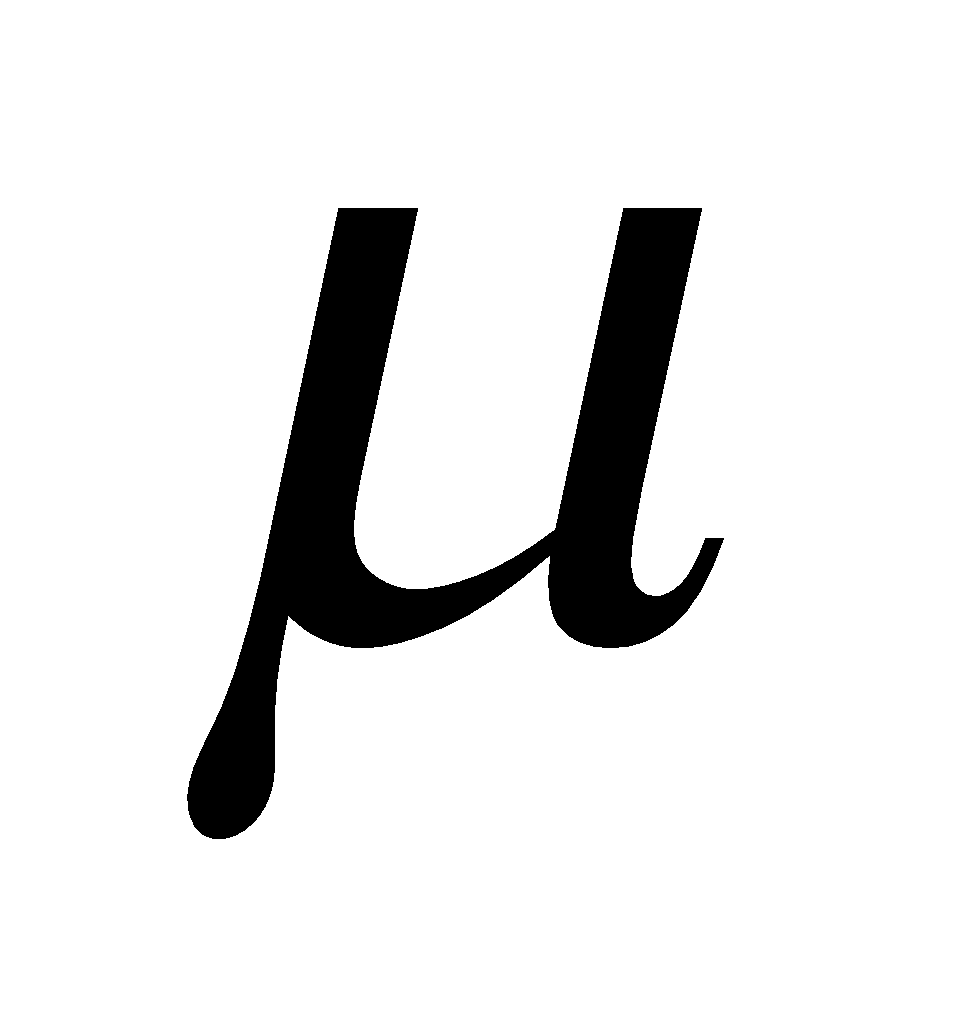 ).
).
The dipole moment is defined as the product of electric charge q and the distance r between the two atoms of a polar molecule. ( 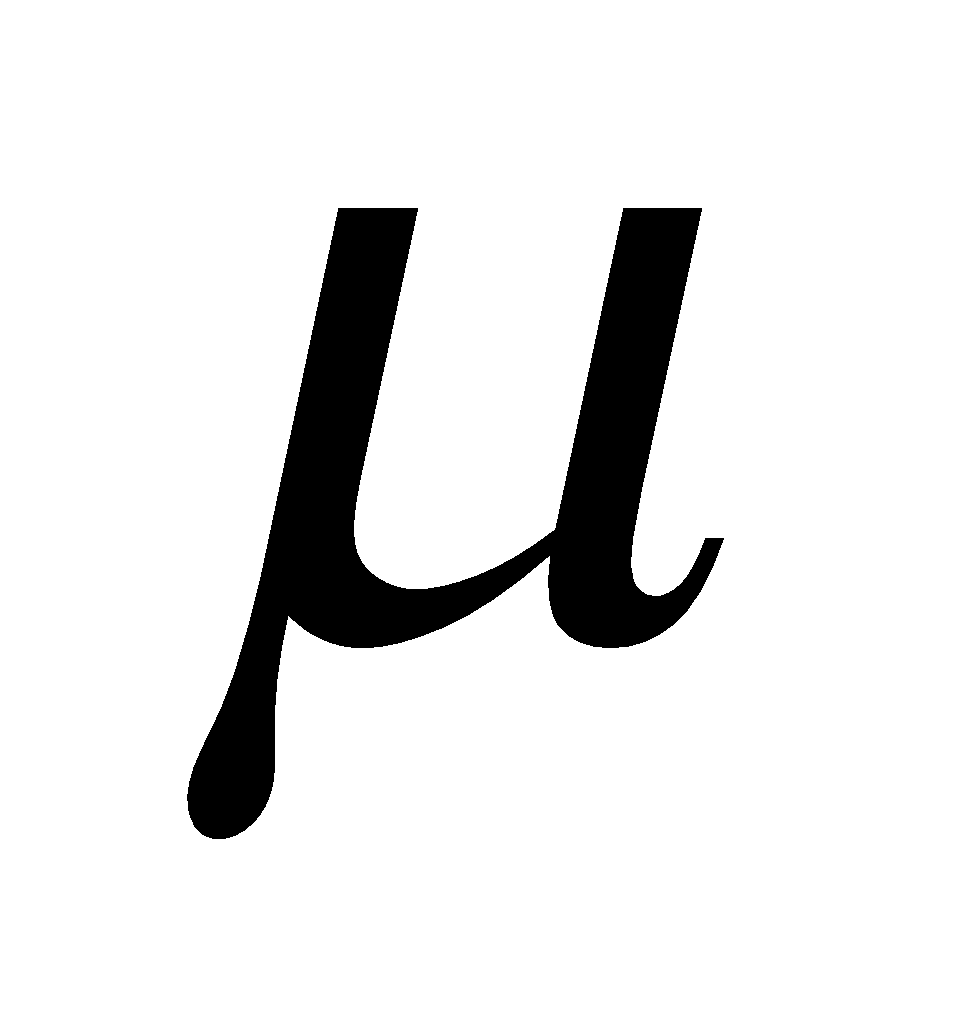 = e × d) Dipole moment is a vector quantity with direction same as that of the line joining positive and negative centres.
= e × d) Dipole moment is a vector quantity with direction same as that of the line joining positive and negative centres.
Thus molecules having dipole moment (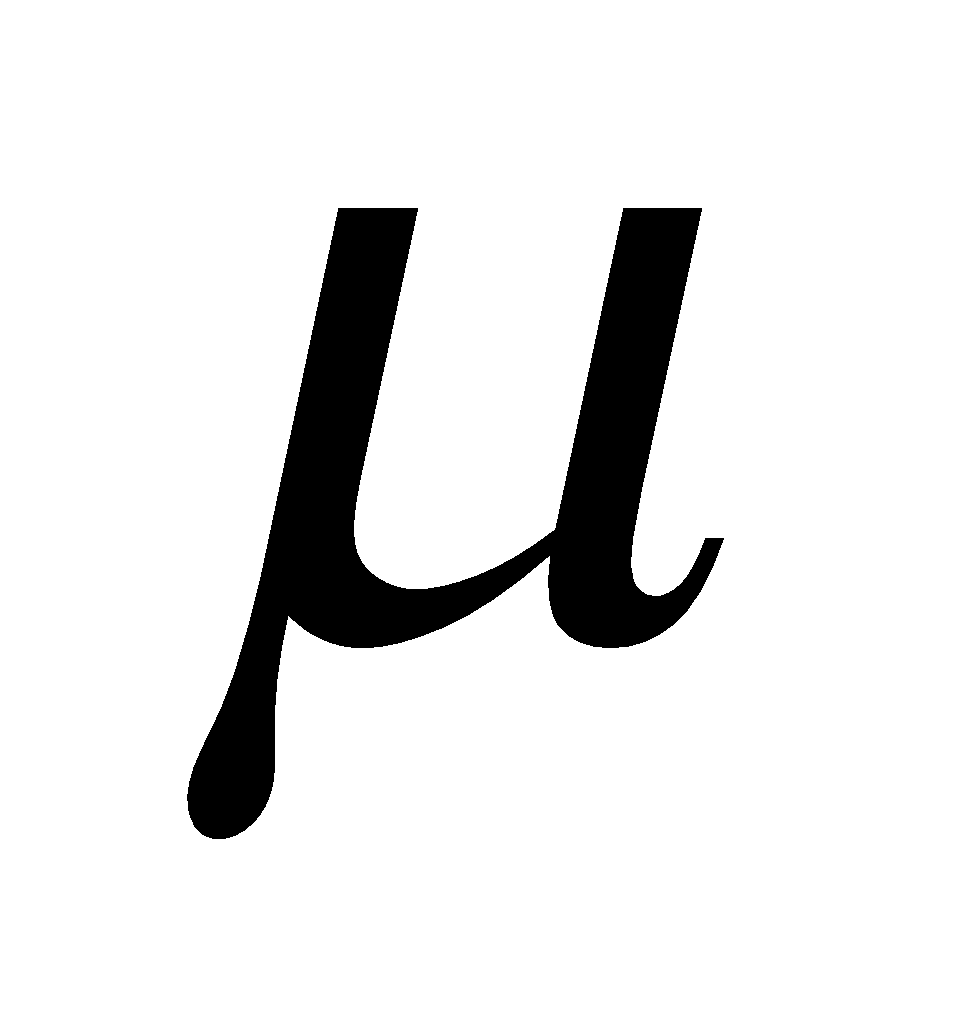 = 0) are called non polar molecules and molecules with
= 0) are called non polar molecules and molecules with 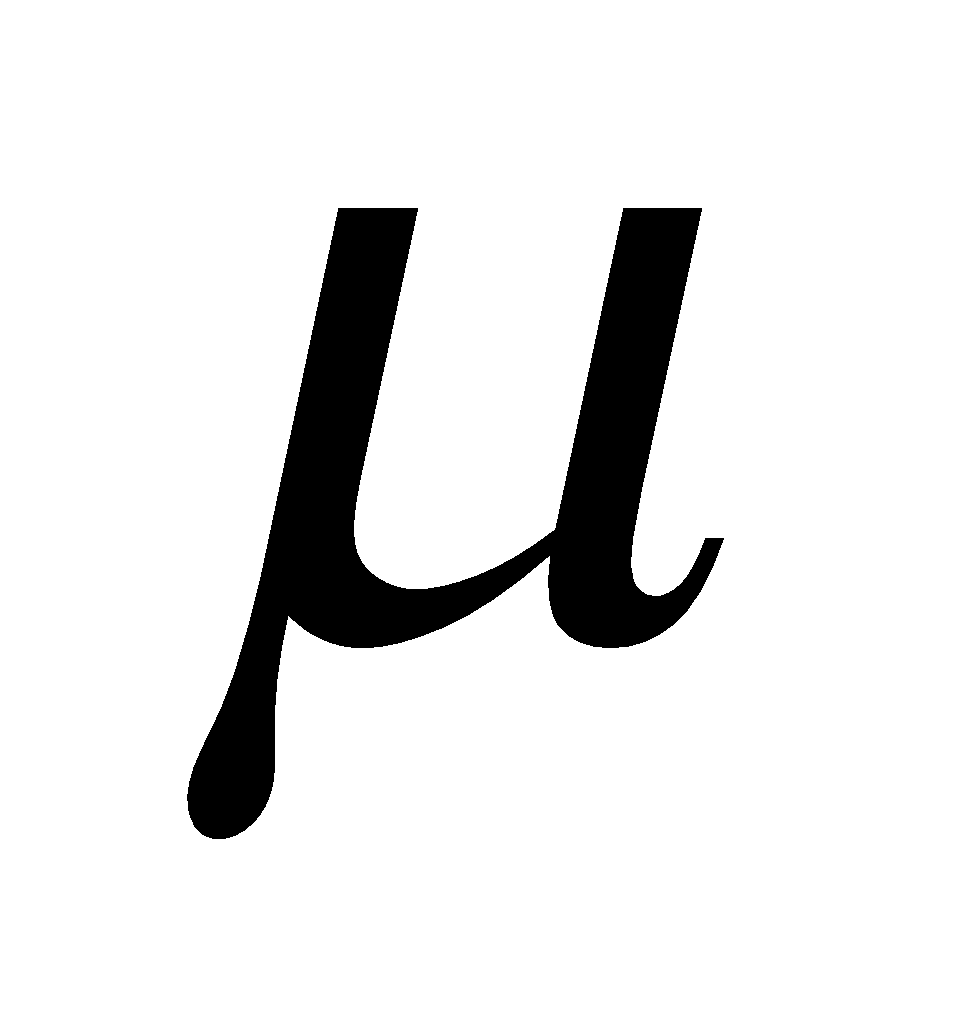 > 0 are polar. Greater is
> 0 are polar. Greater is 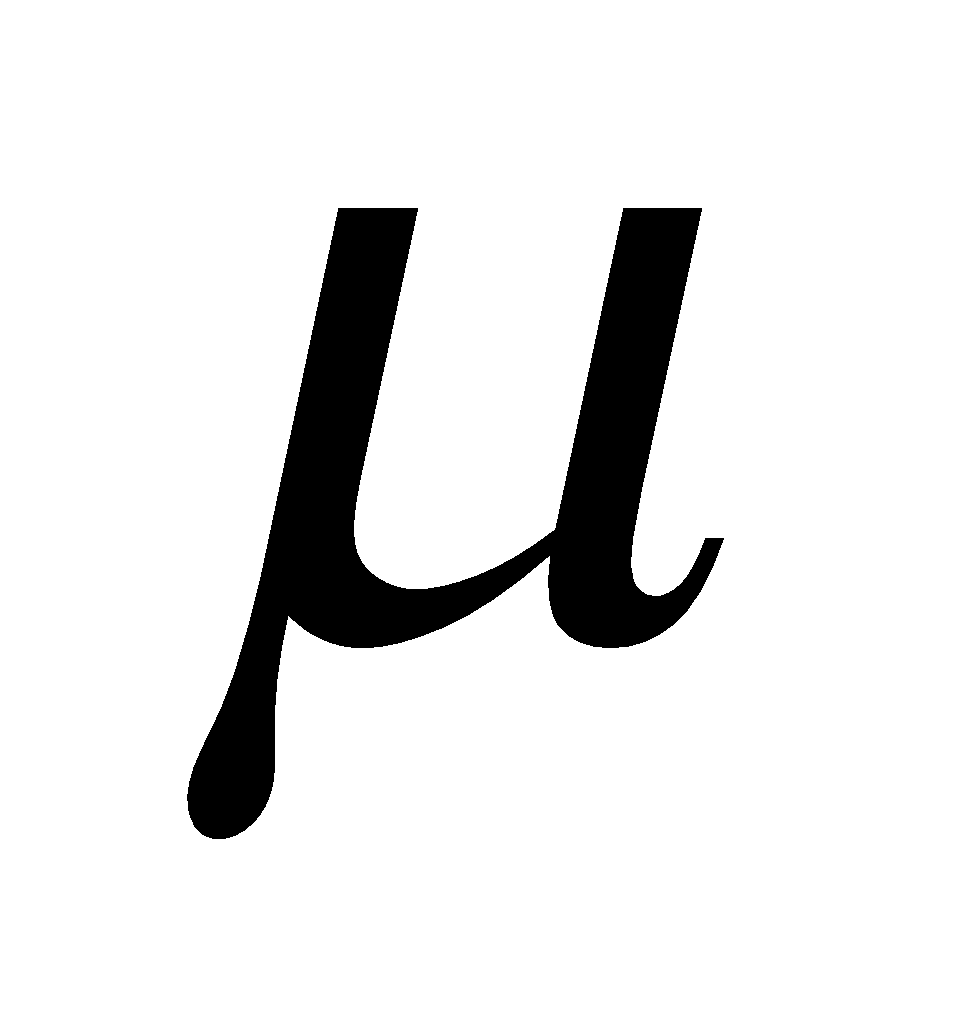 , greater is the polarity.
, greater is the polarity.
For polyatomic molecules with two or more bonds, net dipole moment is the resultant of vector addition of individual moments. For e.g. in the figure shown, the resultant dipole moment,
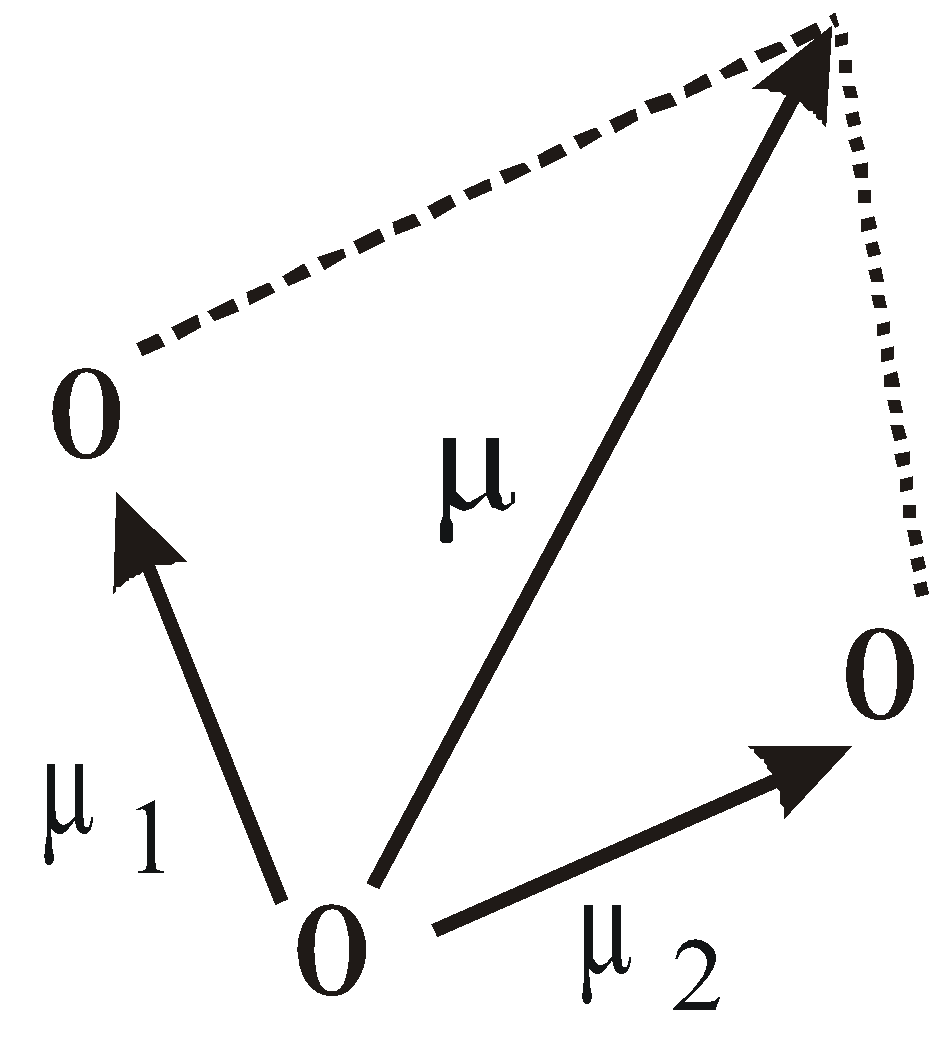
While expressing dipole moments, generally charge is given in electrostatic units (esu) and distance in angstrom units (1Aº =10–10 m). Thus dipole moment of an electron separated from unit positive charge by a distance 1Aº would be (4.80 × 10–10 esu) × (10-8 cm) = 4.8 × 10–18 esu cm = 4.8 Debye.
APPLICATIONS OF DIPOLE MOMENT
- Dipole moment is helpful in predicting the geometry of the molecule.
- Dipole moment helps in determining the polarity.
- Dipole moment can distinguish between symmetrical and non symmetrical molecules. eg. CO2 has 0 dipole moment as it is symmetrical whereas H2O has a dipole moment of 1.85D.
- Cis and trans isomers can be distinguished by dipole moments, usually cis isomers have higher dipole moment and hence higher polarity e.g.
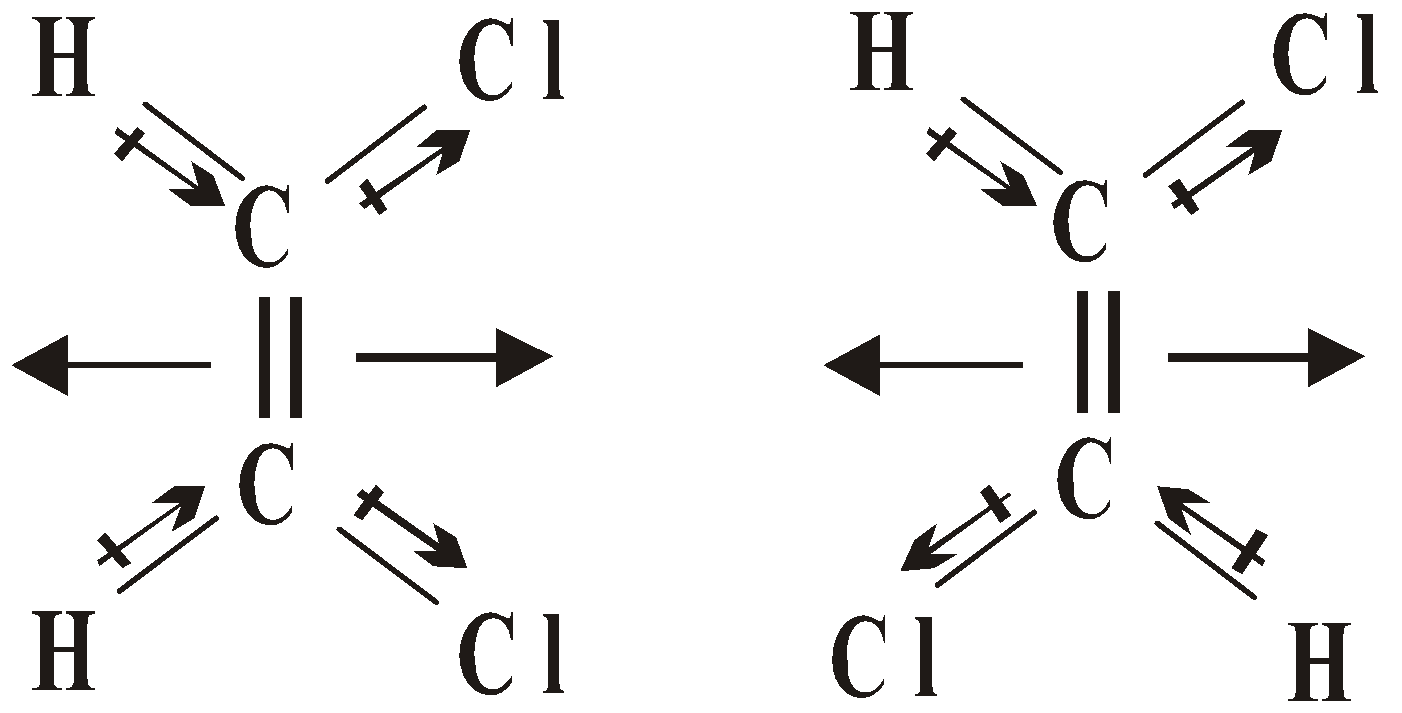
Cis trans
- Dipole moment is greatest for ortho isomer, zero for para isomer and less than that of ortho for meta isomer. o > m > p. e.g.
ortho meta para
- Ionic character can be determined by using dipole moment
For e.g. experimental dipole moment for HCl is 1.03 and 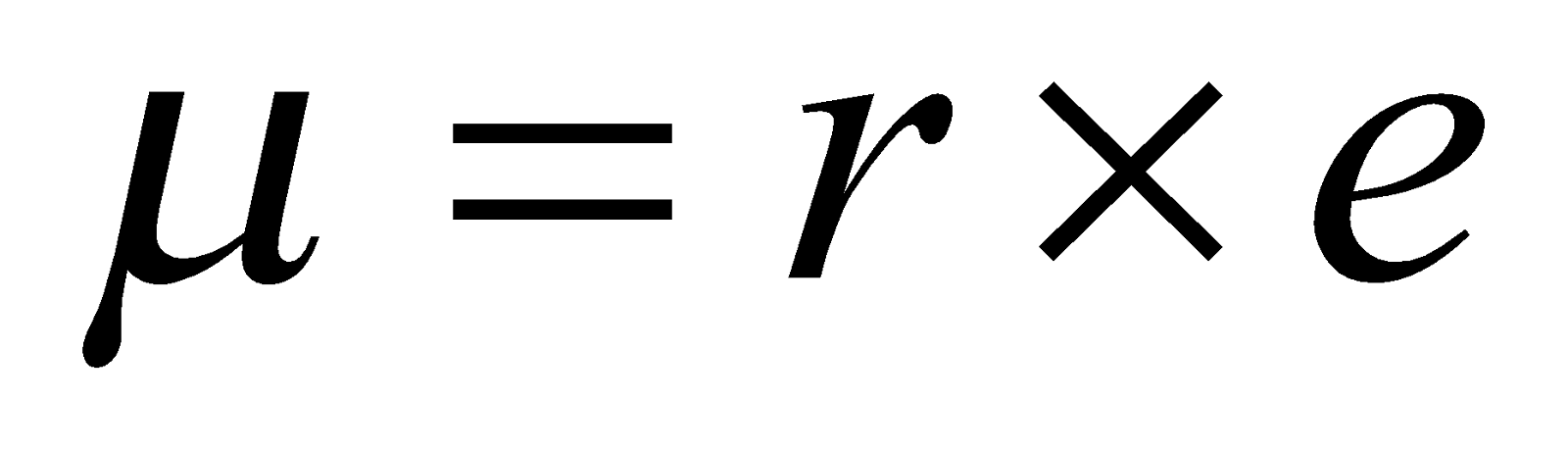
as r = 1.26 and, e = 4.8 × 10-10 esu,
Thus the ionic character
Thus we can say % ionic character
- Hybridisation can be determined by dipole moment for eg.
- If a molecule AB2 has
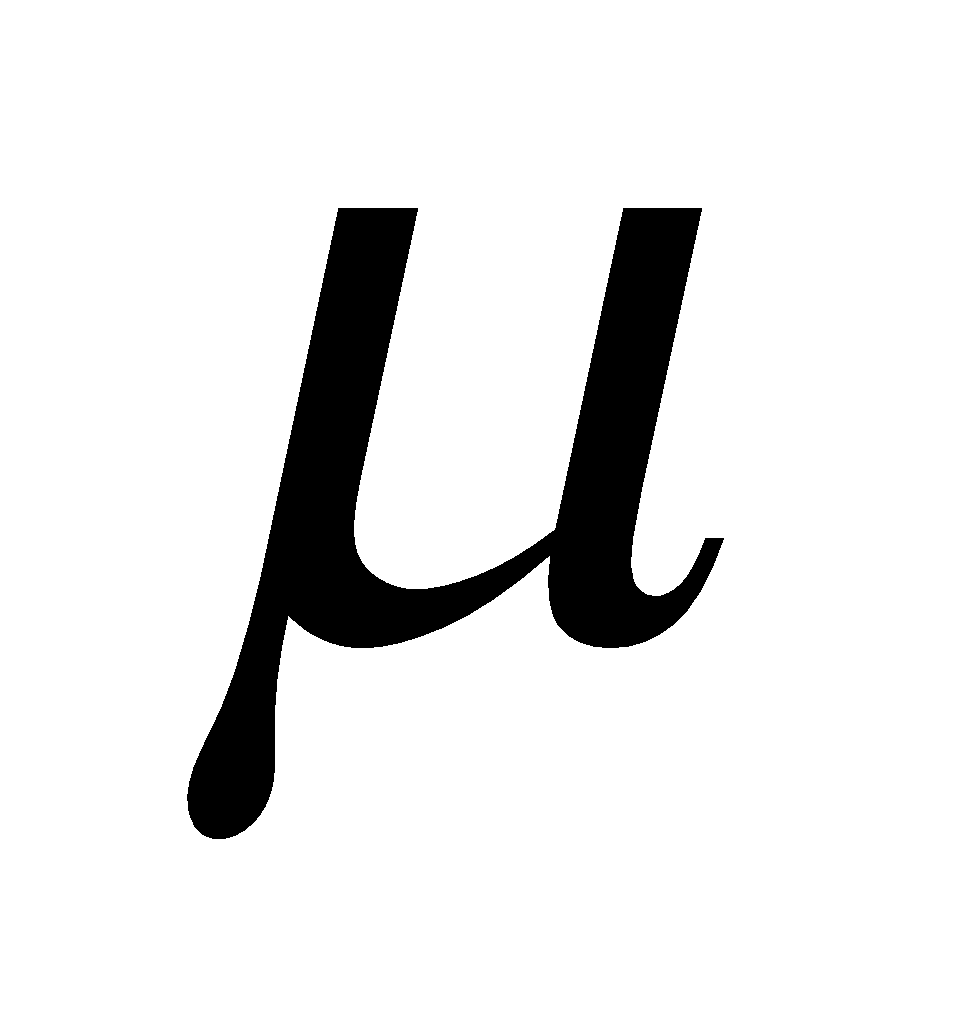 =0, the s orbitals used by
=0, the s orbitals used by
A (z < 21) must be sp hybridised e.g. BeF2 - If a molecule AB3 has
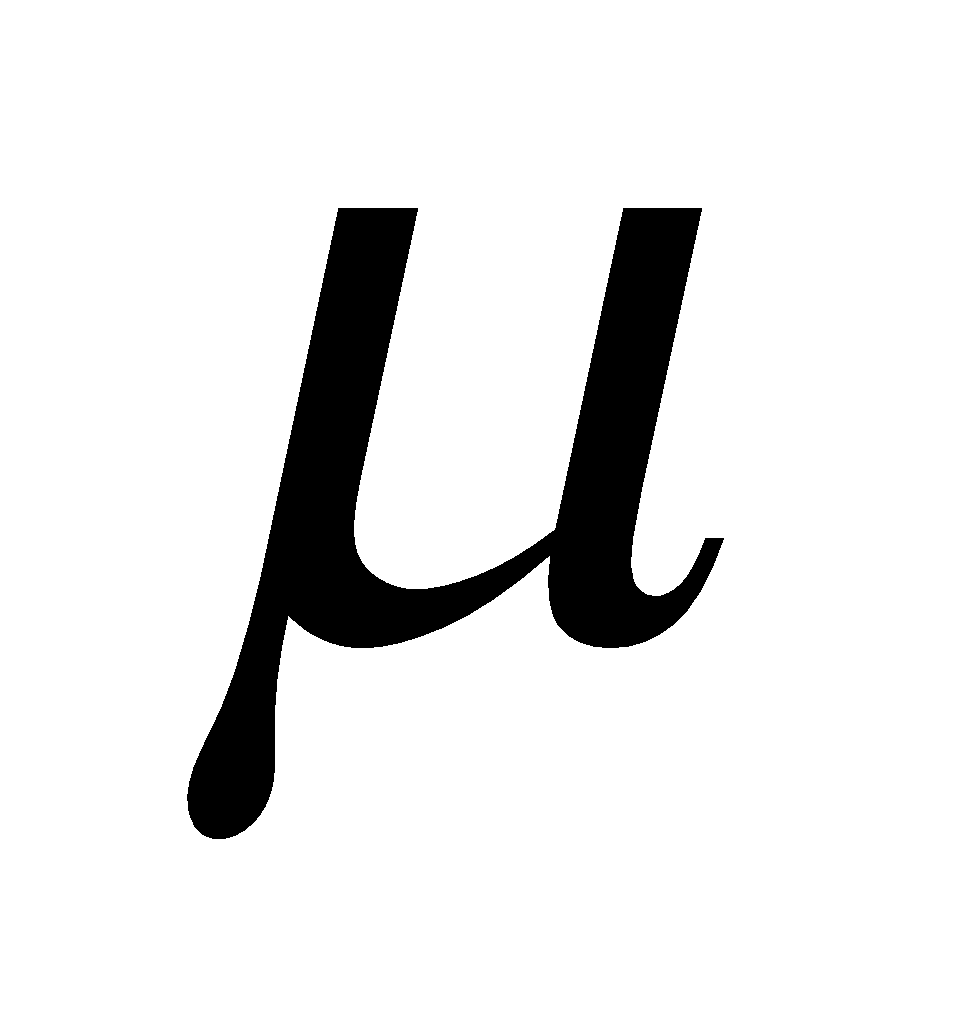 =0, the s orbitals used by
=0, the s orbitals used by
A (z < 21) must be sp2 hybridised e.g. BF3 - If a molecule AB4 has
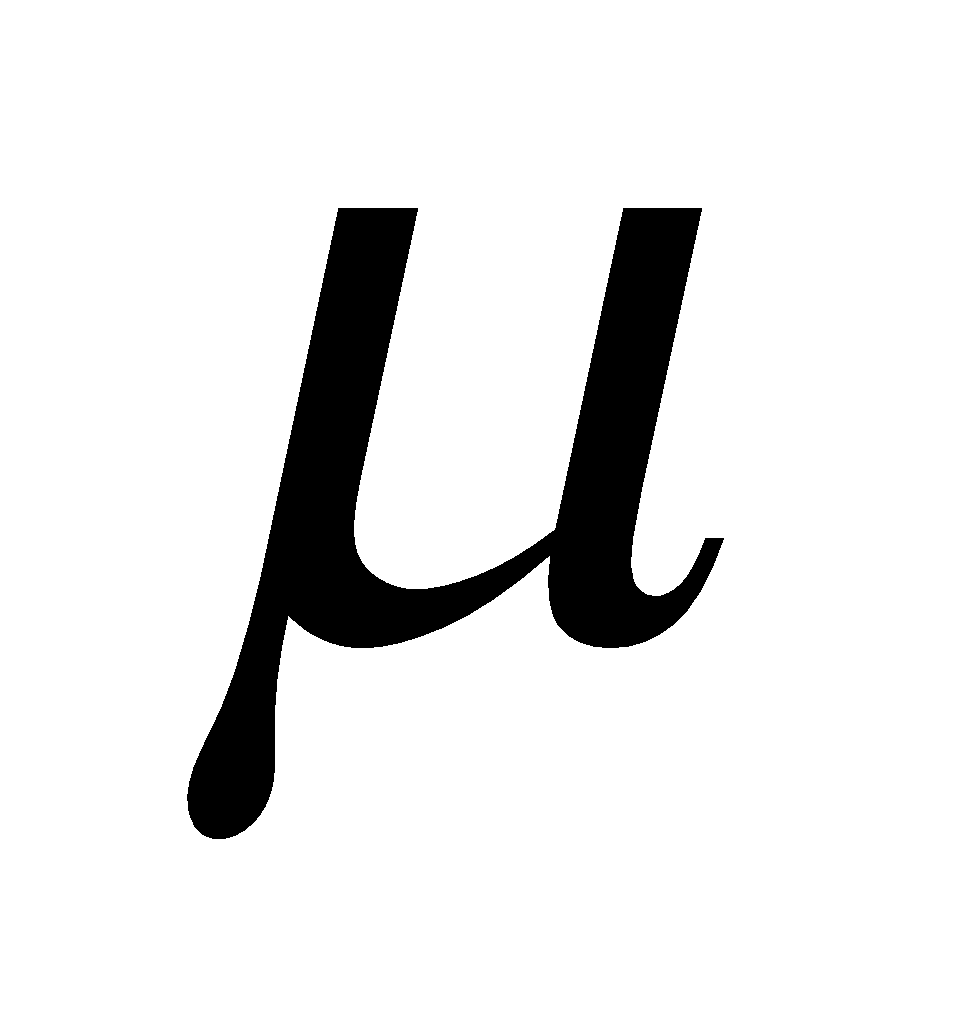 =0, the s orbitals used by
=0, the s orbitals used by
A (z < 21) must be sp3 hybridised e.g. CCl4
BOND LENGTH
It is the distance between the nuclei of two atoms between which the bond is formed. Other names for bond length are interatomic distance or bond distance. It is usually expressed in angstrom unit (1Å=10-10m). Pauling’s rule states that for covalently bonded atoms, bond length is sum of covalent radii. Thus for a covalent compound AB, the bond length is rA + rB. where rA and rB are covalent radii of A and B
BOND LENGTH DEPENDS ON
- Bond order – Bond length decreases with bond order. For e.g. C – C length in CH3 – CH3 is 1.54 Å, C = C length in CH2 = CH2 is 1.34 Å and CC length in CHCH is 1.20 Å. Larger the s character, smaller is bond length.
- Electronegativity – If one of the atoms is more electronegative than other, the bond length is found to be smaller than expected value. The relation that holds true for most compounds is
rAB = rA + rB – 0.09 (xA – xB)
where xA and xB are electronegativities of A and B
- Hybridisation – Larger is s character, shorter is orbital and hence shorter is the length of bond formed by it.
- Resonance and delocalisation – C-C bond length is 1.54Å and C = C bond length is 1.34 Å but in benzene, due to resonance, the carbon-carbon bond is neither single nor double but intermediate between single and double and same holds for bond length and is equal to 1.39 Å.
BOND ENERGY
Bond strength or bond energy is the amount of energy required to break a bond in one gram mole of a substance in gaseous state. Bond energy is also called bond dissociation energy. Its units are kJmol–1. Bond dissociation energy and (average) bond energy are same in diatomic molecules as they have only one bond. But in molecules having many bonds, bond dissociation energy of the bonds are different and bond energy is the average value of bond dissociation energy
FACTORS AFFECTING THE BOND ENERGY
- Atomic size – Strength of shorter bond is more e.g. atomic size of Cl, Br, I has the order Cl < Br < I and their bond energies, Cl – Cl (243 kJ mol-1) > Br – Br (192 kJ mol–1)
> I – I(151 kJmol–1) - Bond order – Higher bond order implies higher energy e.g. bond energies for and are 347.2, 610.0 and 835.1 kJ mol-1 respectively.
- Electronegativity – Larger the difference in electronegativities of bonded atoms, more is the attraction, shorter is bond length and hence higher is bond energy.
- Hybridisation – Due to directional character of hybridised orbitals, extent of overlapping increases and hence stronger bonds are formed.
- Type or extent of overlapping – Coaxial overlappings (s-s, s-p, p-p) have higher extents and hence are stronger than collateral overlappings. Thus sigma bond (coaxial overlapping) is stronger than pi bond (collateral overlapping).As p orbitals are more directional, p – p coaxial overlapping gives stronger bond in comparison to s – s overlapping.
- Repulsion between lone pair – In a bonding atom having lone pair the bond formed is weaker due to non localisation of lone pair. Mutual repulsion takes place between lone pair clouds of two atoms, decrease the strength and hence the bond energy. For e.g. as lone pair at each C – C is 0, N – N is one, O – O is two, so values of their bond energies are in order C – C > N – N >O – O.
DETERMINATION OF BOND ENERGY
Bond energy is determined by thermochemical methods by determining either the heat of atomisation i.e. heat required to break the molecule into its atoms or by the heat of formation of compound.
BOND ANGLE
Bond angle is the angle between two adjacent bonds in a molecule.
Thus bond angles in CO2 and H2O are 180º and 105º.
FACTORS AFFECTING BOND ANGLE
- Electronegativity – Cl – O- Cl bond angle in Cl2O is more than F – O – F bond angle in OF2 because in Cl2O, O atom is more electronegative compared to Cl atom due to which shared electrons are attracted towards O. This makes bonded electrons of two Cl-O bonds to come close and hence more repulsion resulting in wider bond angle. Whereas in OF2, F is more electronegative than O thus bonded electrons of F-O bond are closer to F atom. This makes bonded electrons of two O – F bonds to go apart, feeling less repulsion and hence a lower value for bond angle.
- Hybridisation – sp3 hybridisation at central atom implies a bond angle of 109º 28¢ each e.g. CH4, CH3CH3 etc. sp2 hybridisation leads to 120º bond angle as in BF3. sp hybridisation makes angle 180º e.g. BeCl2.
- Lone pair repulsions – Presence of lone pair at central atom results in repulsion of shared pair of electrons. Thus bonds at central atom are displaced inside making the value of bond angle smaller e.g. bond angle in ammonia is 107º even though hybridisation at nitrogen is sp3. It is due to presence of lone pair of electrons at nitrogen atom which causes more repulsion between N and H and hence decreasing the bond angle. Bond angle can be measured by X-ray techniques or by I.R. spectroscopy.
Baeyer’s strain theory states that any shift in normal bond angle causes strain over the molecule making it less stable and more reactive. Thus CH4, C2H6 are quite stable while ethylene is less stable (bond angle 120º).
OCTET RULE
During formation of a covalent bond, the atoms attain an inert gas electronic configuration (ns2p6 configuration). This is known as Octet rule.
EXCEPTIONS OF OCTET RULE
- Incomplete octet : Consider the following molecules
- Expansion of octet : Consider the following molecules
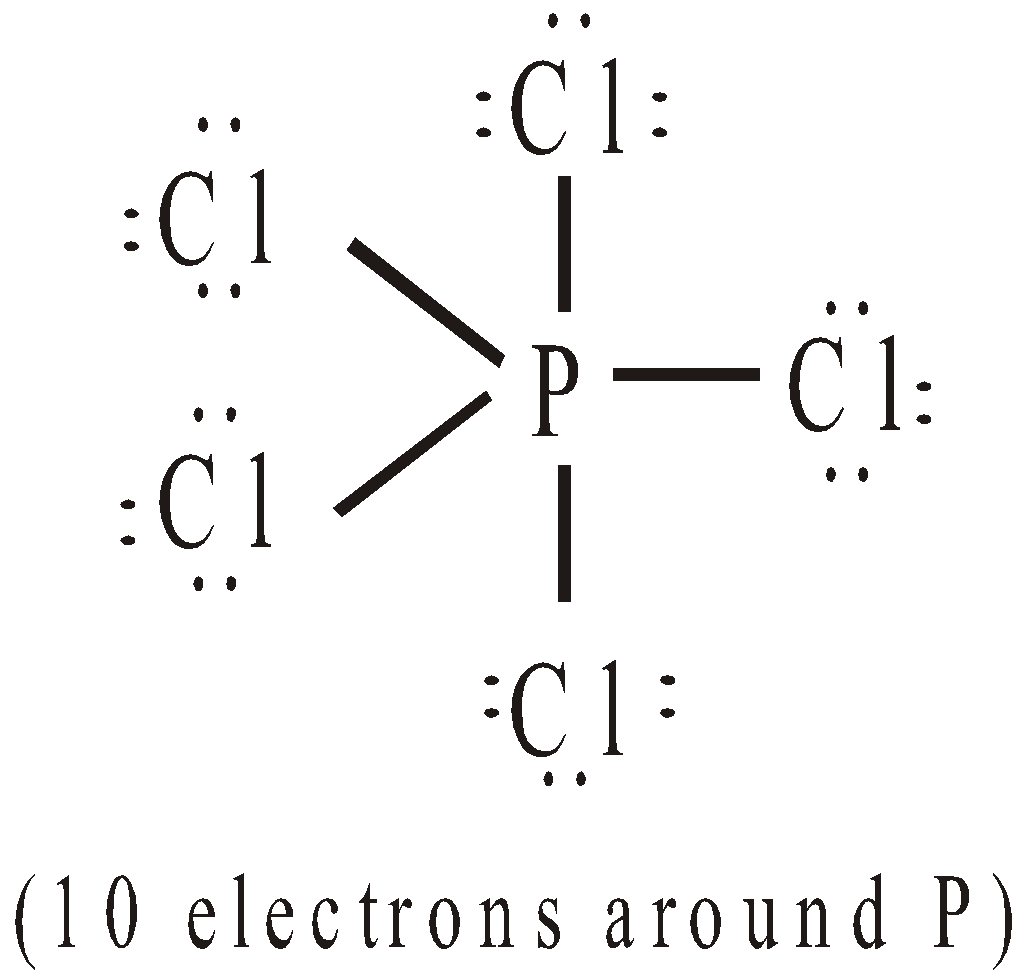
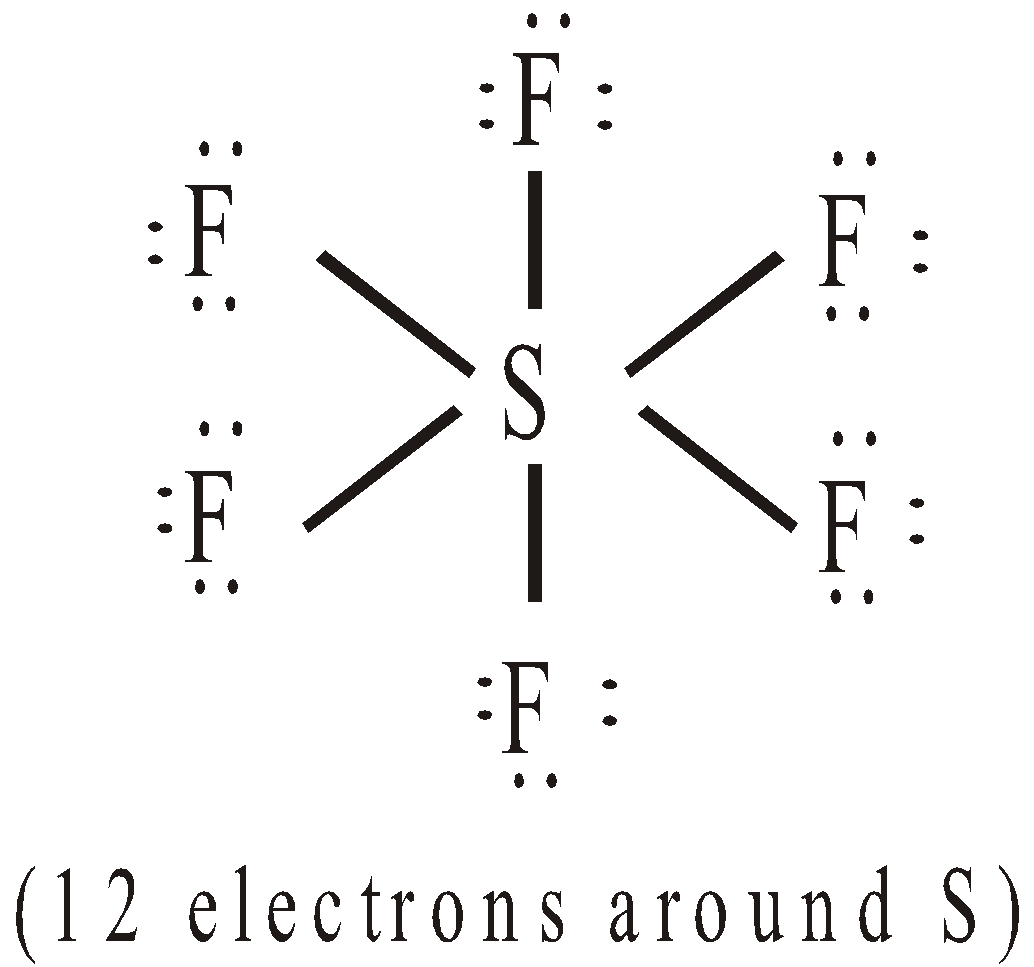
EXPLANATION FOR THE FAILURE OF OCTET RULE
The concepts given below explain the failure of the octet rule :
- Sidwick’s rule of maximum covalency : The maximum covalency of an element depends on the period to which it belongs and octet can be exceeded for n = 1 it is 2, for n = 2 it is 4, for n = 3 it is 6 and for n > 4 it is 8.
- Sugden’s view of singlet linkage : According to Sugden the octet rule is never violated. There can be one electron bond known as Singlet linkage, Singlet or half bond is formed by sharing of one electron between two atoms.
It is represented by half arrow ( ). Thus SF6 and PCl5 have the structures:
). Thus SF6 and PCl5 have the structures: