7.1 Equilibrium
Essential Idea:
Many reactions are reversible. These reactions will reach a state of equilibrium when the rates of the forward and reverse reaction are equal. The position
of equilibrium can be controlled by changing the conditions
Understandings:
- A state of equilibrium is reached in a closed system when the rates of the forward and reverse reactions are equal.
- The equilibrium law describes how the equilibrium constant (Kc) can be determined for a particular chemical reaction.
- The magnitude of the equilibrium constant indicates the extent of a reaction at equilibrium and is temperature dependent.
- The reaction quotient (Q) measures the relative amount of products and reactants present during a reaction at a particular point in time. Q is the equilibrium expression with non-equilibrium concentrations. The position of the equilibrium changes with changes in concentration, pressure, and temperature.
- A catalyst has no effect on the position of equilibrium or the equilibrium constant.
Applications and Skills:
- The characteristics of chemical and physical systems in a state of equilibrium.
- Deduction of the equilibrium constant expression (Kc) from an equation for a homogeneous reaction.
- Determination of the relationship between different equilibrium constants (Kc) for the same reaction at the same temperature.
- Application of Le Châtelier’s principle to predict the qualitative effects of changes of temperature, pressure and concentration on the position of equilibrium and on the value of the equilibrium constant.
CHEMICAL EQUILIBRIUM
REVERSIBLE REACTIONS
Reactions which do not always proceed to completion and may be made to proceed in the opposite direction under suitable conditions are called reversible reactions e.g.
3Fe + 4H2O  Fe3O4 + 4H2
Fe3O4 + 4H2
H2 + I2  2HI
2HI
N2O4  2NO2
2NO2
N2 + 3H2 2NH3
2NH3
IRREVERSIBLE REACTIONS
Reactions which always proceed to completion in one direction only are called irreversible reactions.
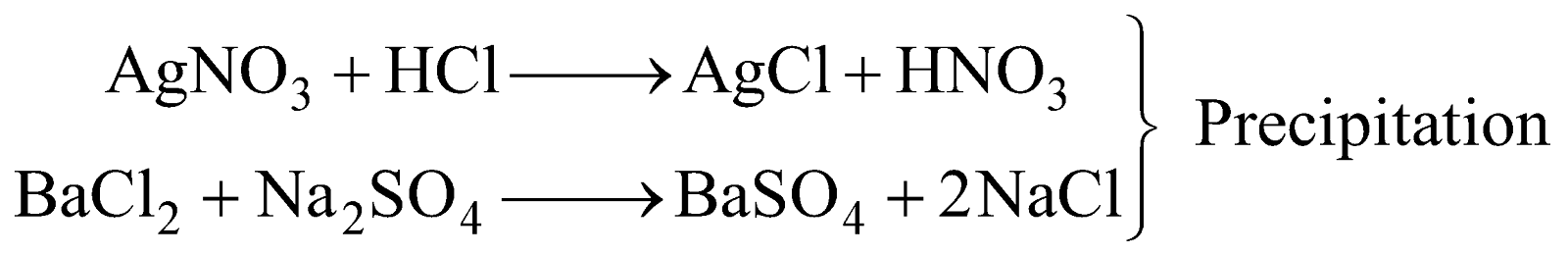
CHEMICAL EQUILIBRIUM
When a reversible reaction is carried out in a closed vessel a stage reached when the speed of the forward reaction equals the speed of the backward reaction and chemical equilibrium is said to be established.
CHARACTERISTICS OF CHEMICAL EQUILIBRIUM
- Equilibrium can be attained from either side.
- Equilibrium is dynamic in nature i.e. at equilibrium, reaction does not stop.
- At equilibrium there is no change in the concentration of various species.
- The equilibrium state remains unaffected by the presence of catalyst. Catalyst helps to attain the equilibrium state rapidly.
- It can be achieved in a closed container.
- The observable properties of the process become constant and remain unchanged.
EQUILIBRIUM STATE AND FREE ENERGY CHANGE
At equilibrium  G is equal to zero and we have
G is equal to zero and we have
LAW OF MASS ACTION
It was put forward by Guldberg and Waage. The law states that the rate at which a substance reacts is directly proportional to its active mass and the rate of a chemical reaction is directly proportional to the product of the active masses of the reacting substances. For a general reaction
aA + bB  cC + dD
cC + dD
Rate of forward reaction 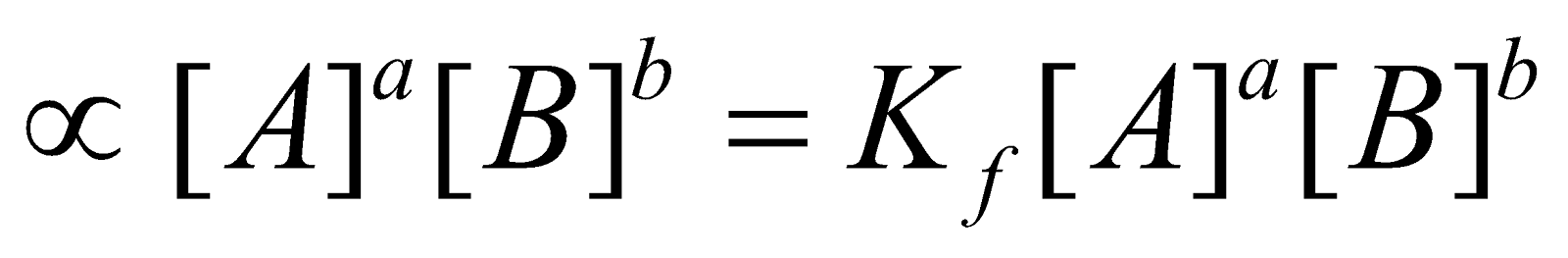
Rate of backward reaction 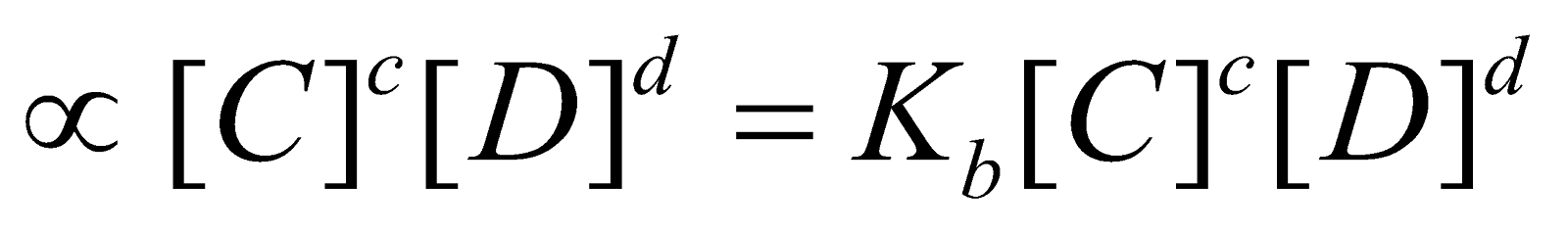
where Kf and Kb are velocity constants for forward and backward reactions respectively. At equilibrium point,
Rate of forward reaction = Rate of backward reaction
Kc is called the equilibrium constant.
FACTORS INFLUENCING EQUILIBRIUM CONSTANT
EQUILIBRIUM CONSTANT IS NOT INFLUENCED BY
- Concentration of reactants and products.
- Presence of a catalyst.
- Pressure.
- Presence of inert materials.
- The direction from which the equilibrium state is reached.
EQUILIBRIUM CONSTANT IS INFLUENCED BY
- Temperature : The variation of equilibrium constant is given by Van’t Hoff equation.
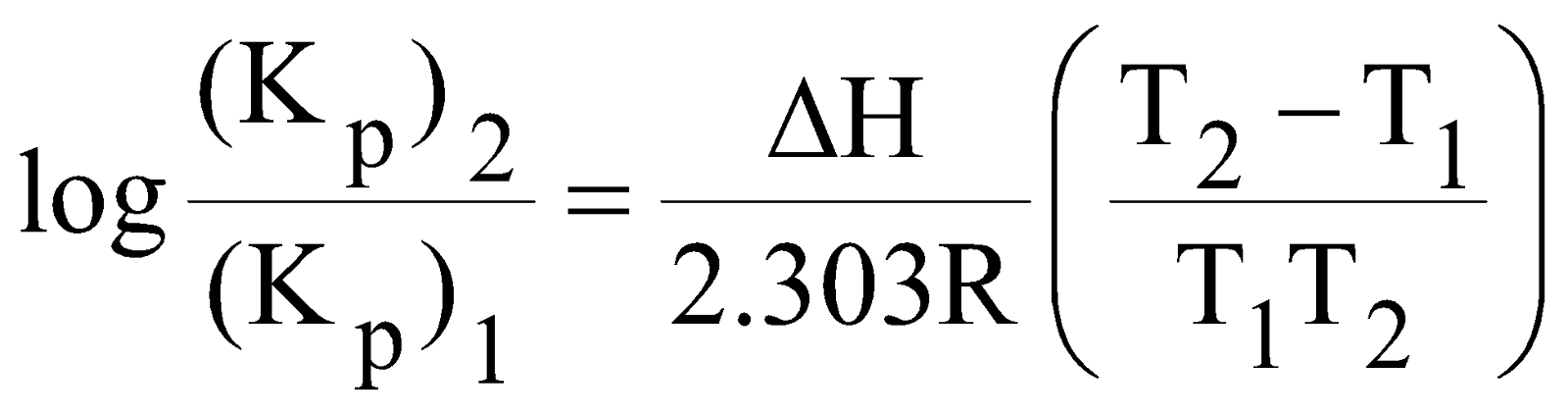
where 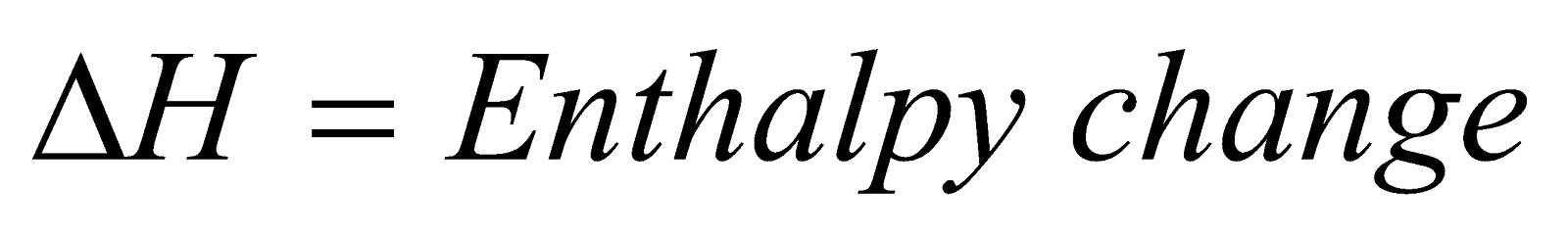 , (Kp)1 and (Kp)2 = Equilibrium Constant at temperature T1 & T2
, (Kp)1 and (Kp)2 = Equilibrium Constant at temperature T1 & T2
R = Universal Gas Constant
For exothermic reaction : Kp decreases with increase of temperature since Kf decreases.
For endothermic reaction : Kp increases with increase of temperature since Kf increases.
- The mode of representing the reaction :
The reaction A + B  C + D may be written as
C + D may be written as
C + D A + B
A + B
- Stoichiometric representation of equation :
N2 + 3H2  2NH3
2NH3
USE OF PARTIAL PRESSURE INSTEAD OF CONCENTRATIONS
For gaseous reacting substances partial pressures are conveniently used since at any fixed temperature partial pressure is directly proportional to concentration. For a general reaction
aA + bB  cC + dD
cC + dD
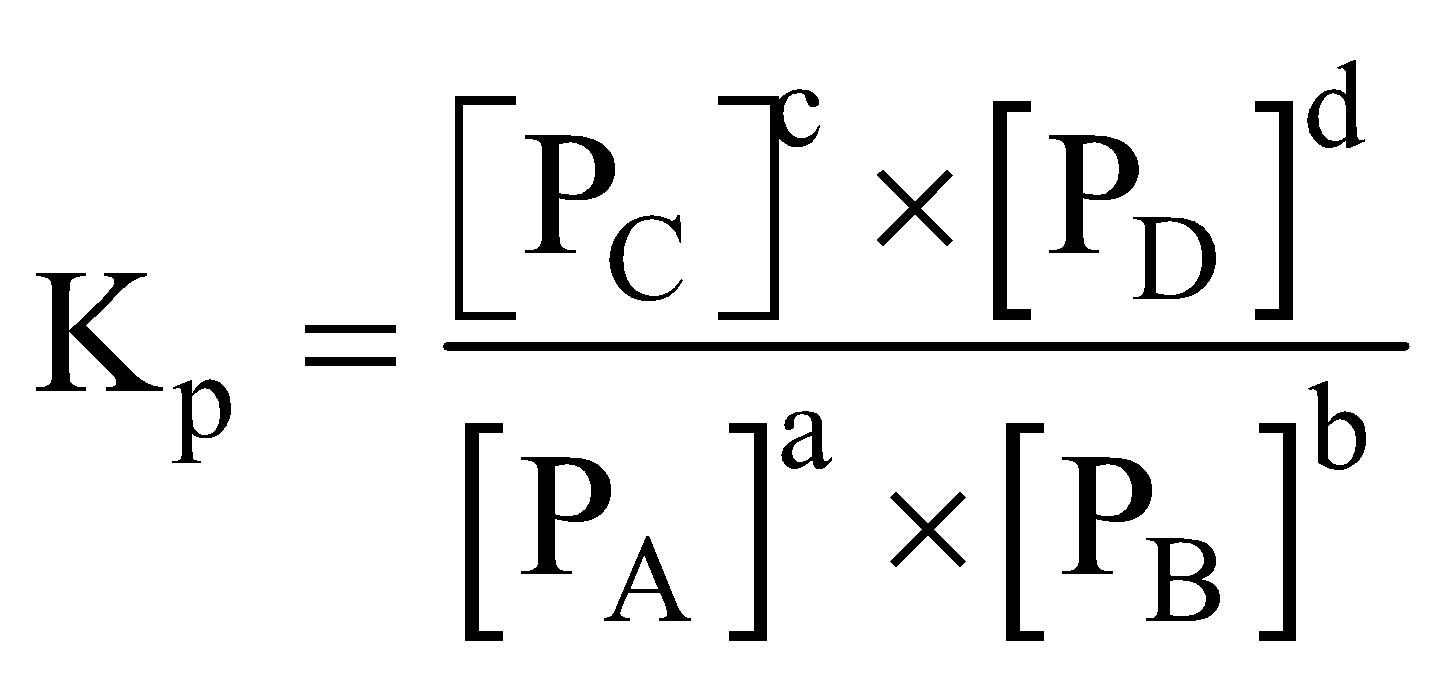
RELATION BETWEEN KC AND KP
where
RELATION BETWEEN KC AND KP FOR DIFFERENT TYPES OF REACTIONS
- When
 n = 0, Kp = Kc e.g. for reaction A
n = 0, Kp = Kc e.g. for reaction A  B.
B.
- When
 n = +ve, Kp > Kc e.g. for reaction A
n = +ve, Kp > Kc e.g. for reaction A 2B.
2B.
- When
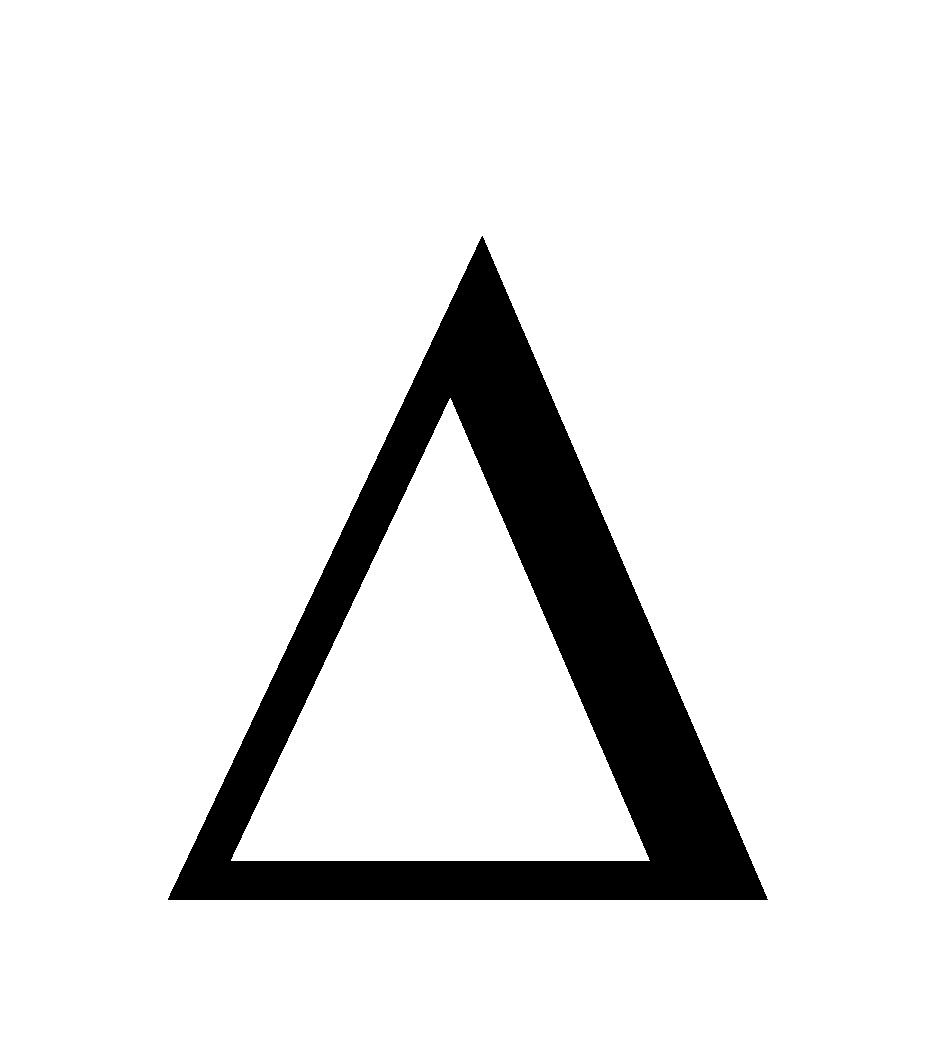
 n = –ve, Kp < Kc e.g. for reaction 2A
n = –ve, Kp < Kc e.g. for reaction 2A B.
B.
UNITS OF Kp AND Kc
- Unit of Kp = (atm)
 n
n - Unit of Kc = (mol lit–1)
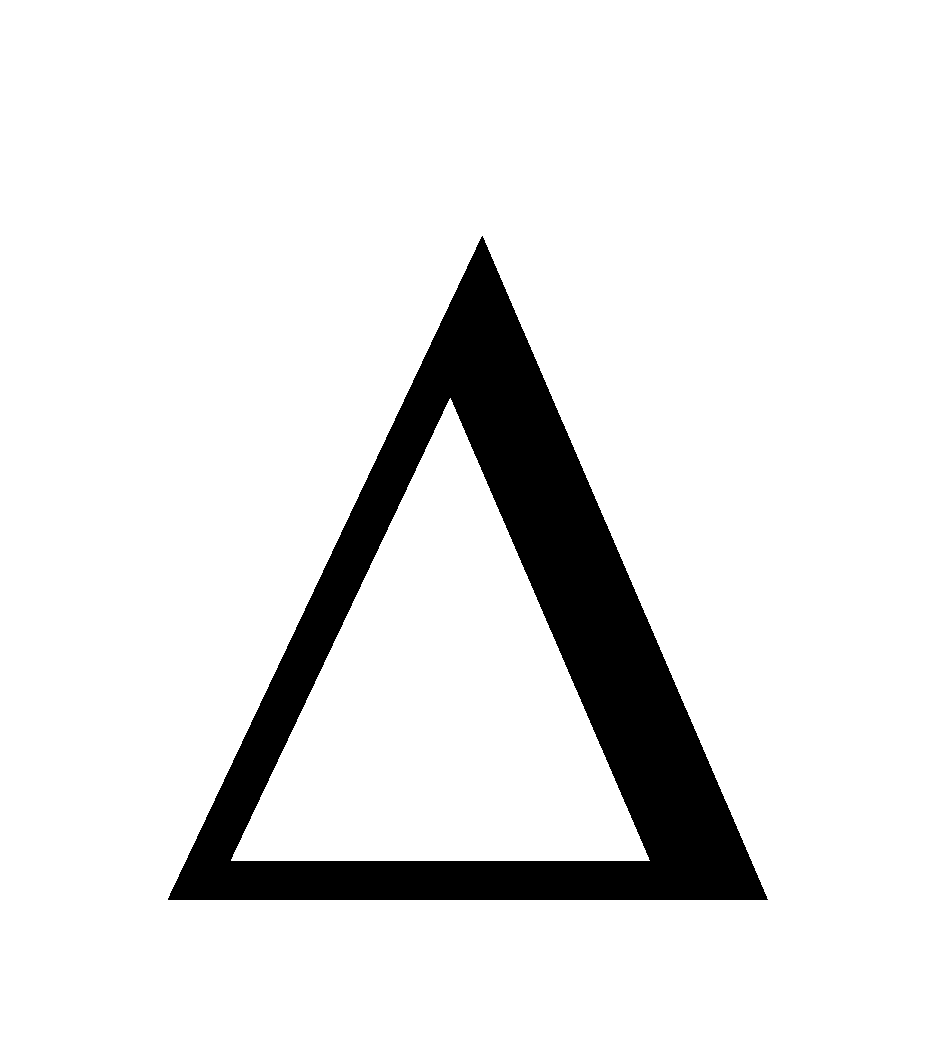 n
n
CHARACTERISTICS OF EQUILIBRIUM CONSTANT
- It has definite value for every chemical reaction at a particular temperature
- The more is the value of Kc or Kp, the more is the completion of reaction or the more is the concentration of products.
- When the reaction can be expressed as sum of two other reactions, the Kc of overall reaction is equal to the product of equilibrium constants of individual reactions.
HOMOGENEOUS EQUILIBRIUM
In homogeneous equilibrium the reactants and products are present in the same phase (gaseous or liquid).
2SO2 (g) + O2 (g)  2SO3 (g)
2SO3 (g)
HETEROGENEOUS EQUILIBRIUM
In heterogeneous equilibrium the reactants and products are present in two or more phases.
3Fe(s) + 4H2O(g)  Fe3O4 (s) + 4H2 (g)
Fe3O4 (s) + 4H2 (g)
CHEMICAL EQUILIBRIUM APPLIED TO HOMOGENEOUS SYSTEM
GASEOUS SYSTEM
They are of two types
- Gaseous reactions in which the number of moles of products remain the same as that of reactants
Hydrogen – iodine equilibrium : Suppose, a moles of H2 and b moles of I2 are present in a container of V litres. At equilibrium x moles of each have combined to form HI.
Initial molar conc. 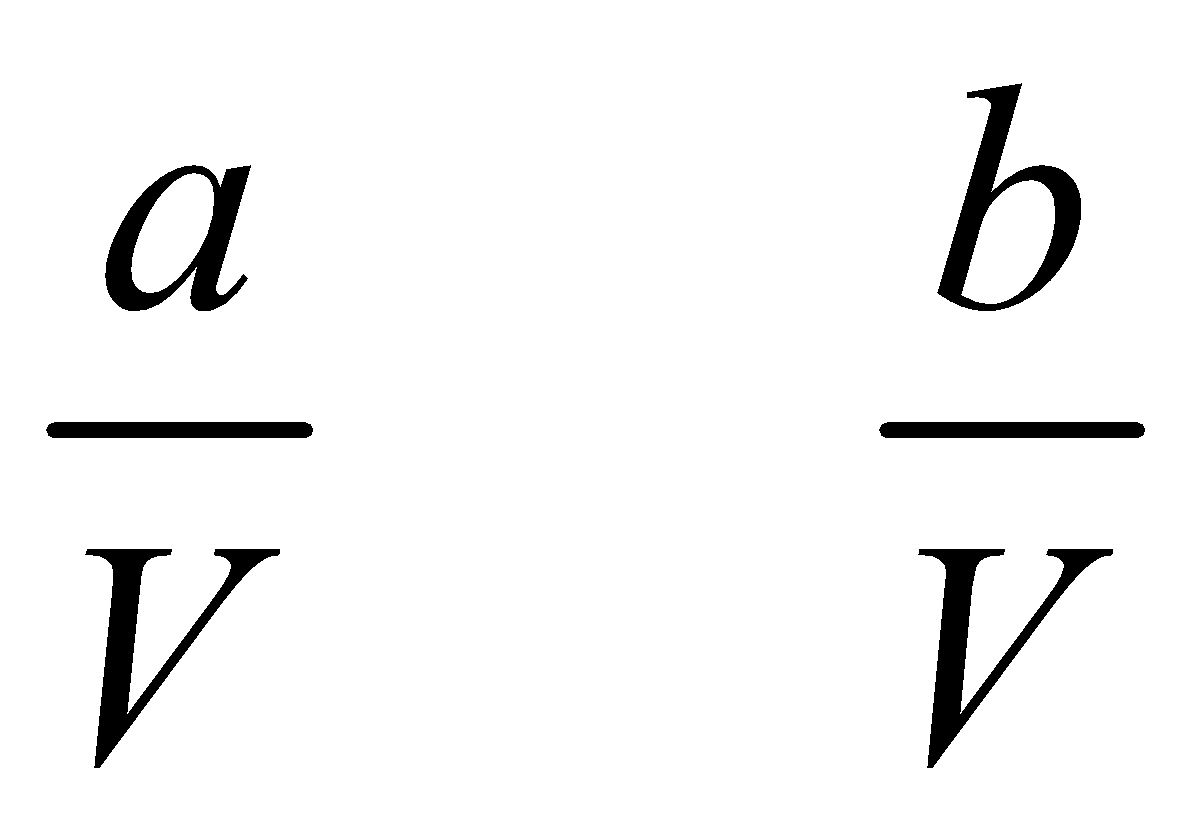 0
0
Eqb. molar conc. 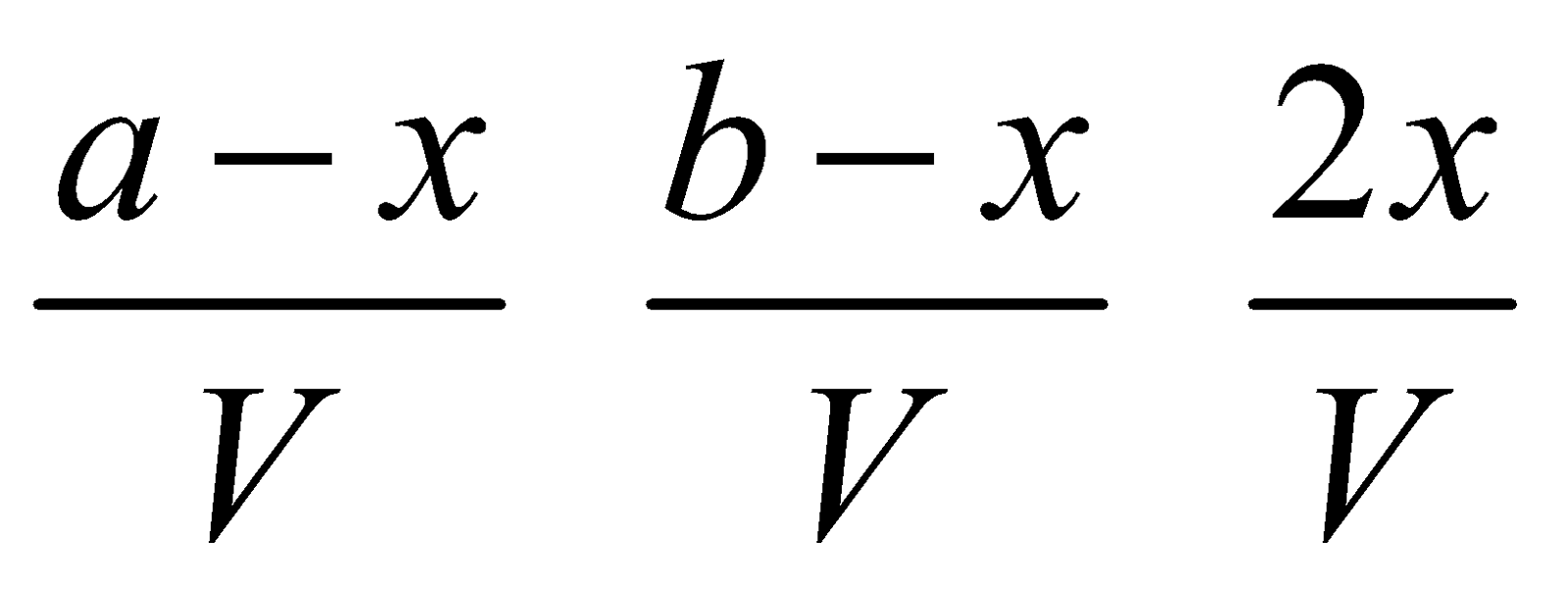
Applying the law of chemical equilibrium
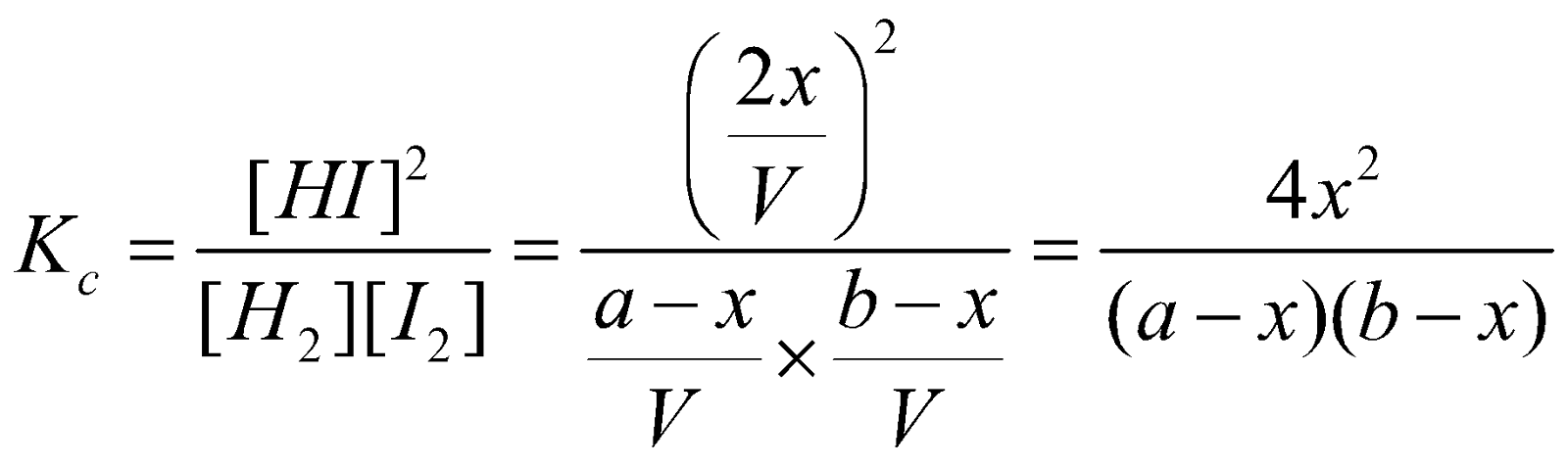 ……….(i)
……….(i)The equilibrium constant written as Kc indicates that active masses are expressed in terms of molar concentrations.
The eq. (i) does not contain the volume term. Thus equilibrium is independent of volume and therefore of pressure.
- Gaseous reactions in which the number of moles of products and reactants are different.
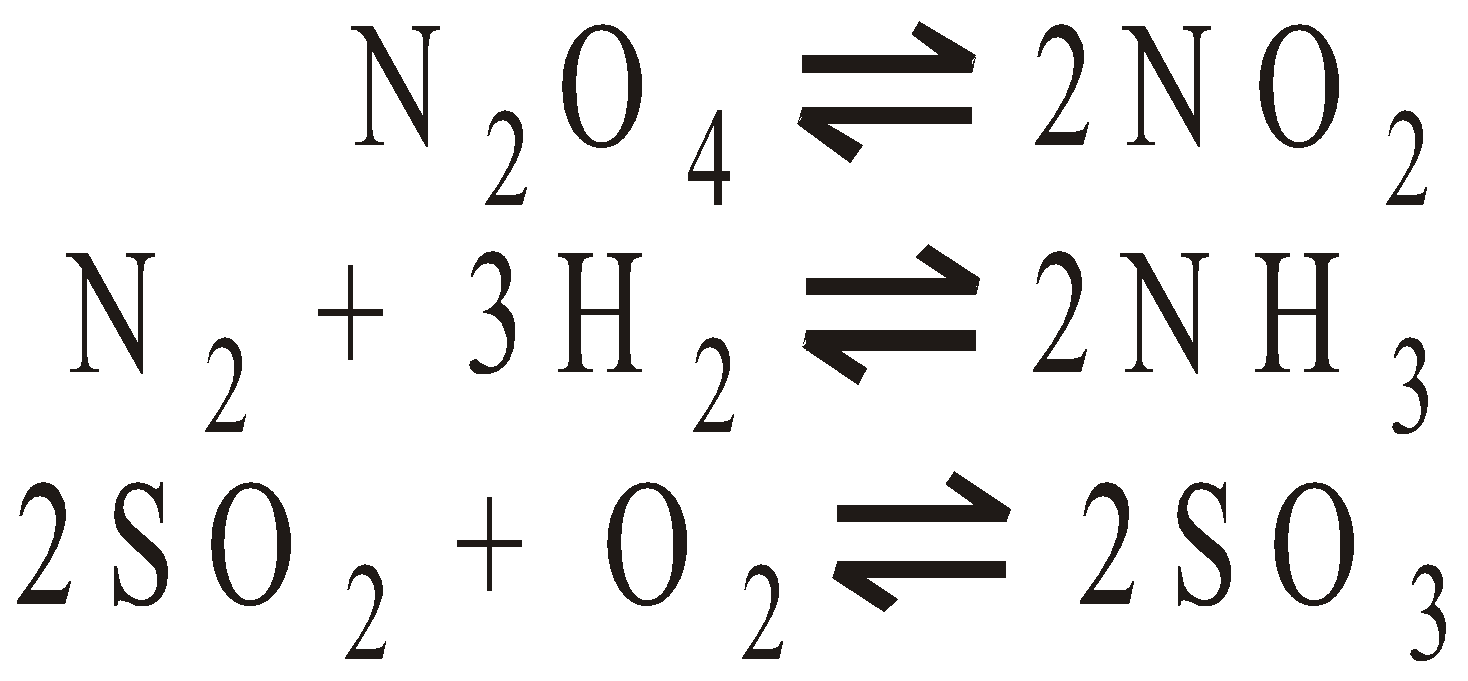
Dissociation of PCl5 : Suppose ‘a’ moles of PCl5 are present in a container of V litres. At equilibrium x moles have dissociated.
Initial molar Conc. 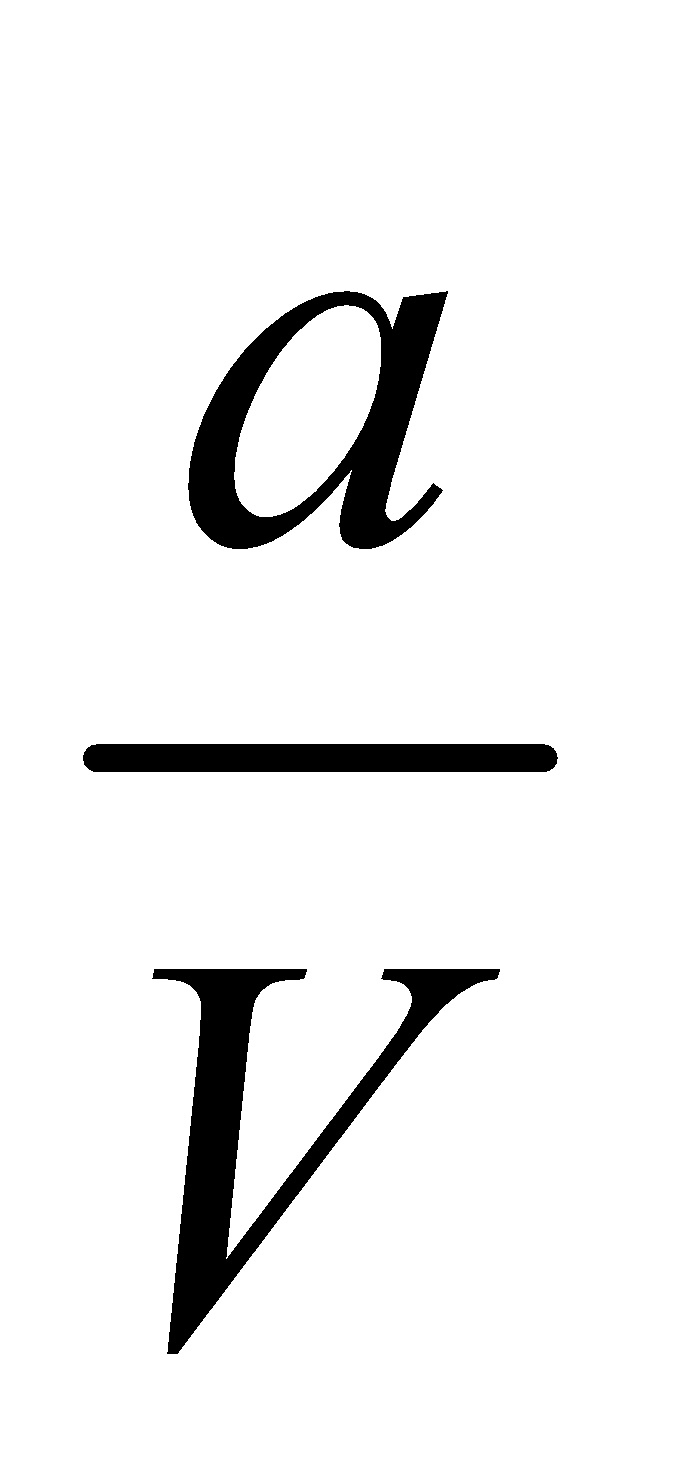 0 0
0 0
Eqb. molar Conc. 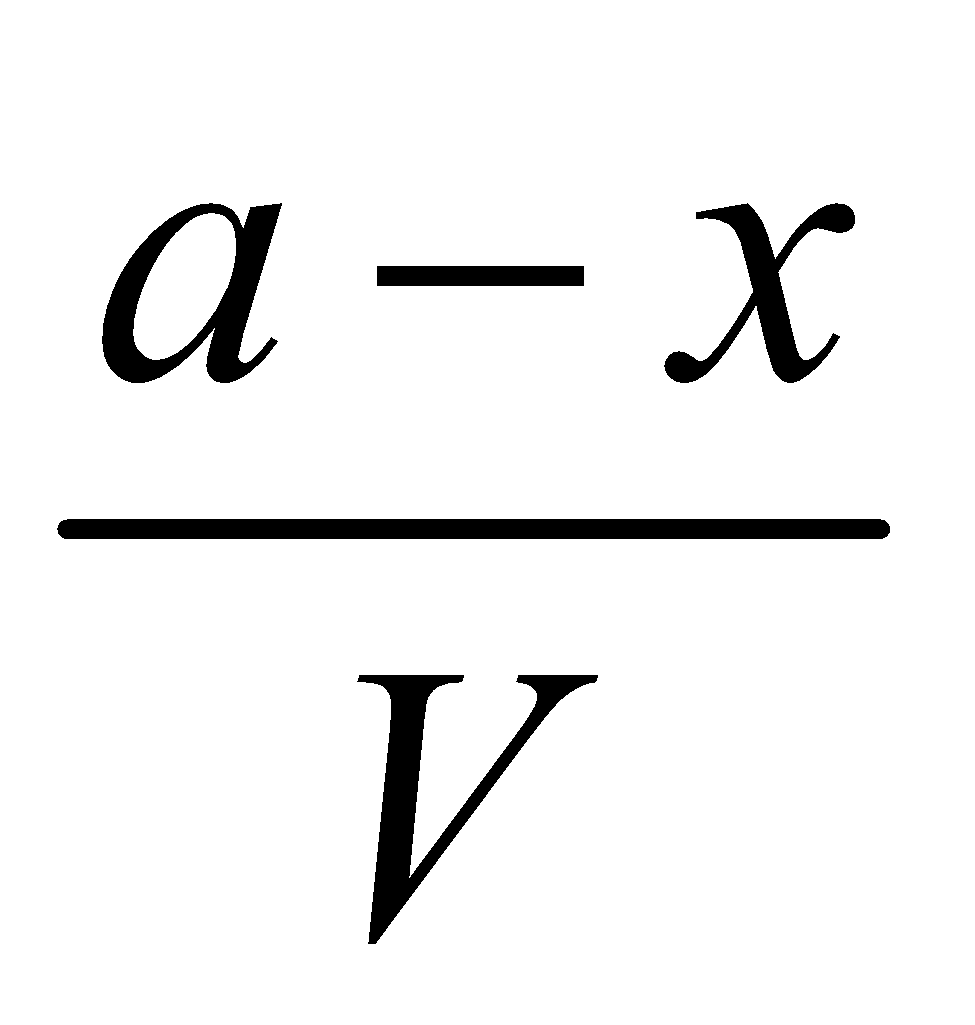
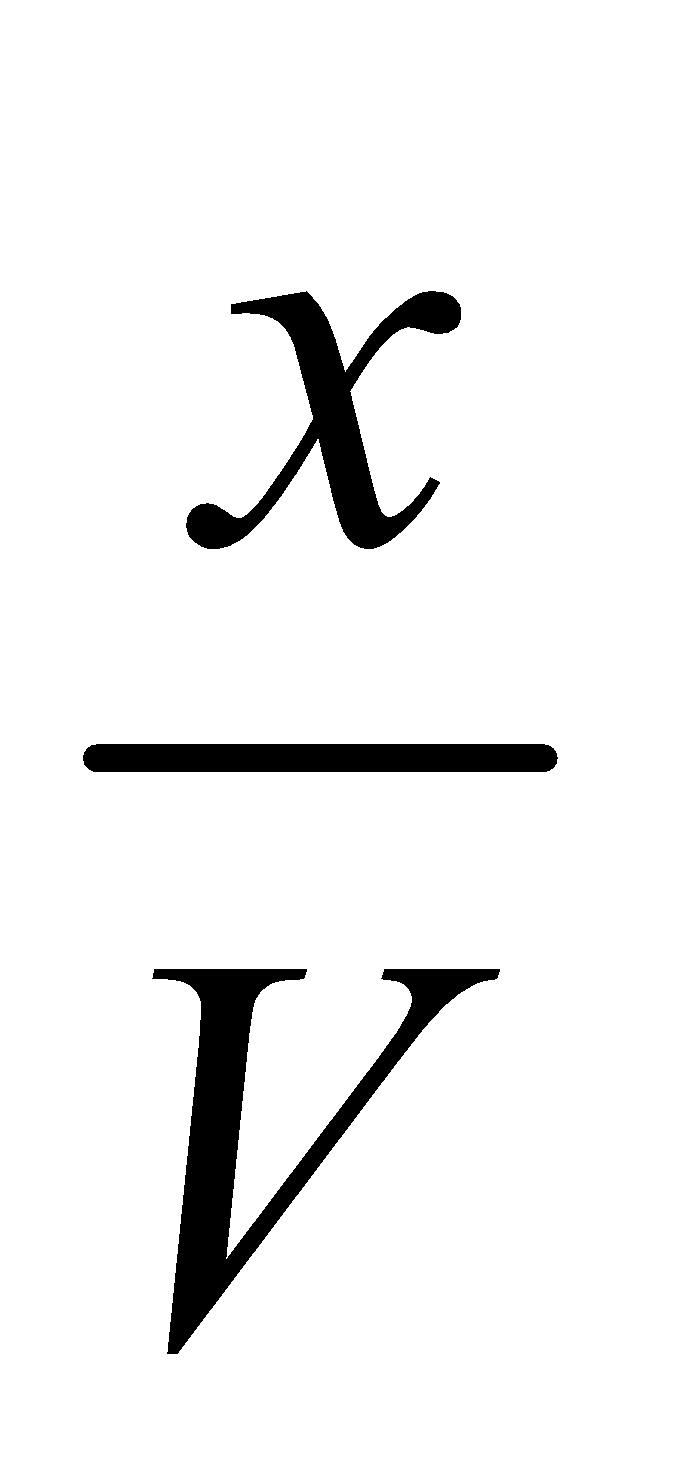
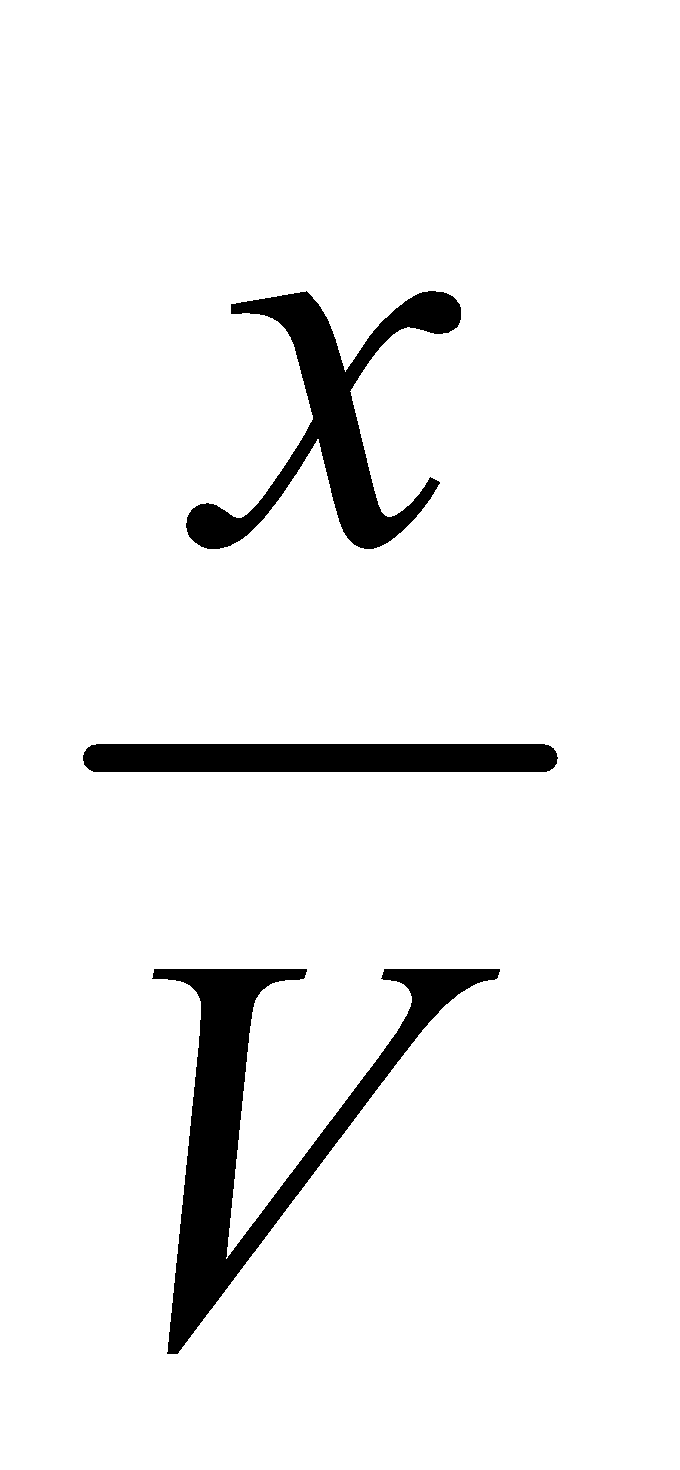
Applying the law of chemical equilibrium
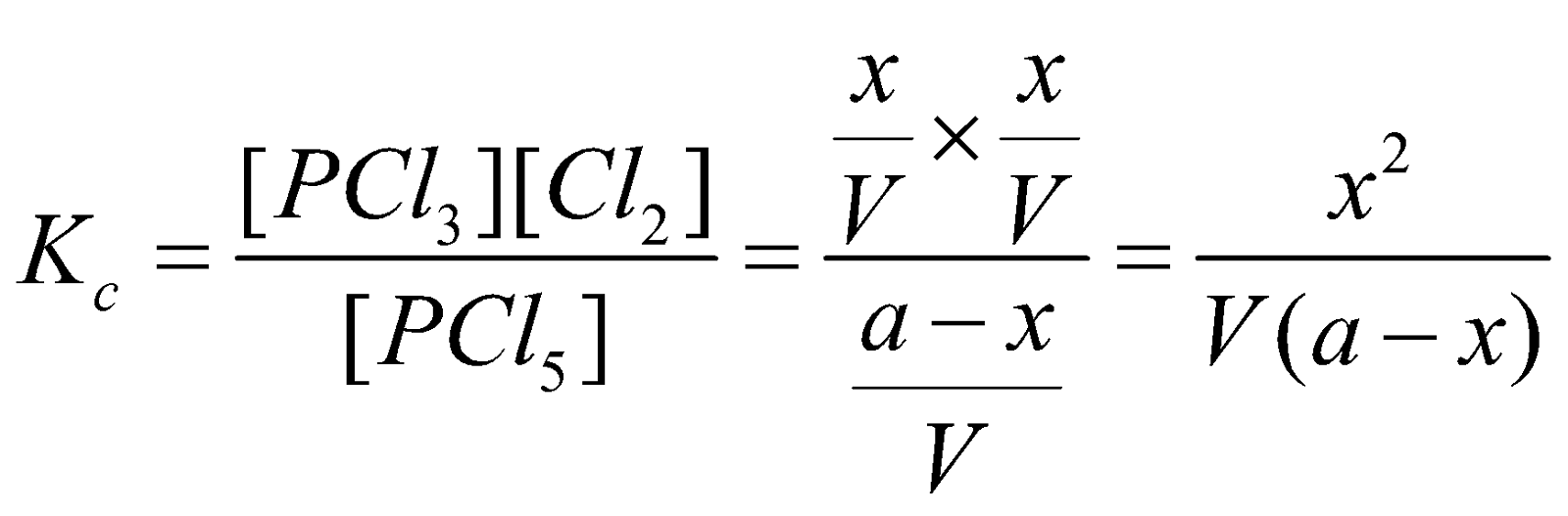 …….(i)
…….(i)The eq.(i) contains the V term in denominator . If volume increases, the dissociation of PCl5 must also increase to keep Kc constant. The decrease of pressure will cause increase in volume and so the dissociation.
If the value of x is small then 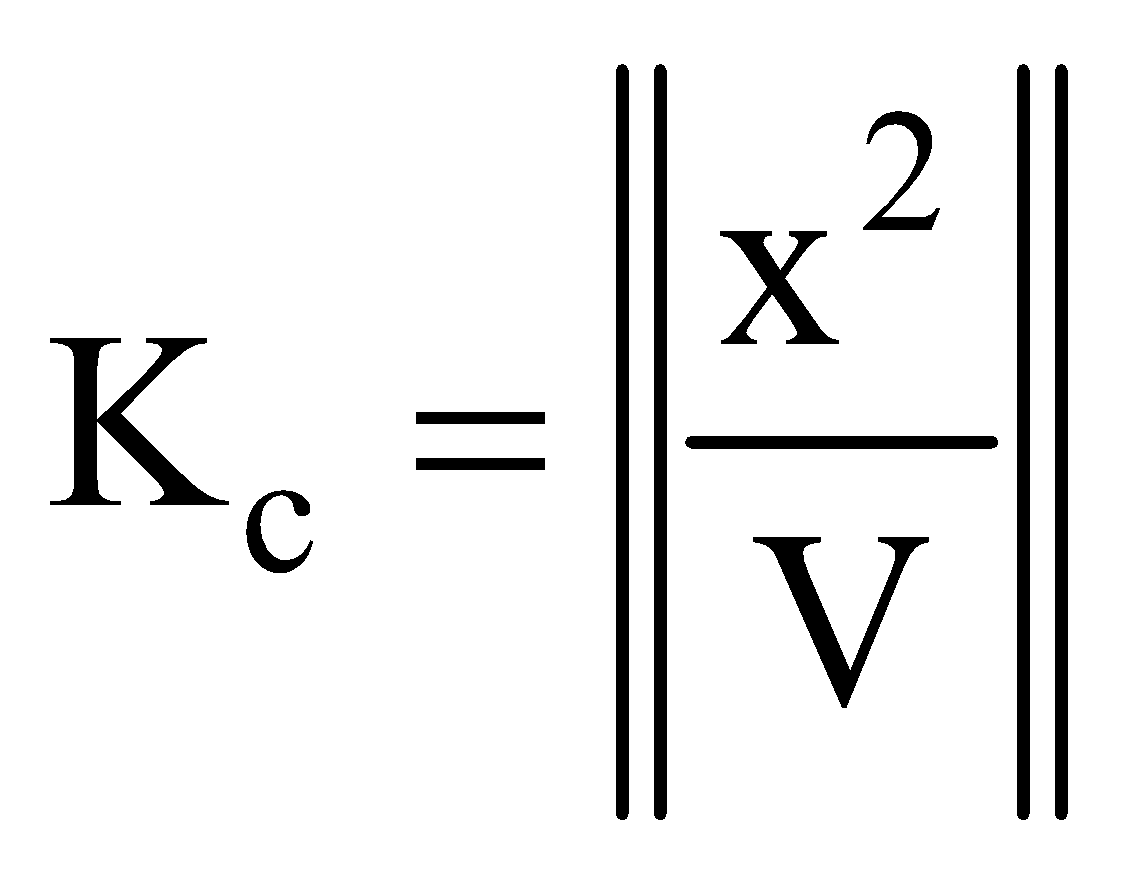 ;
; 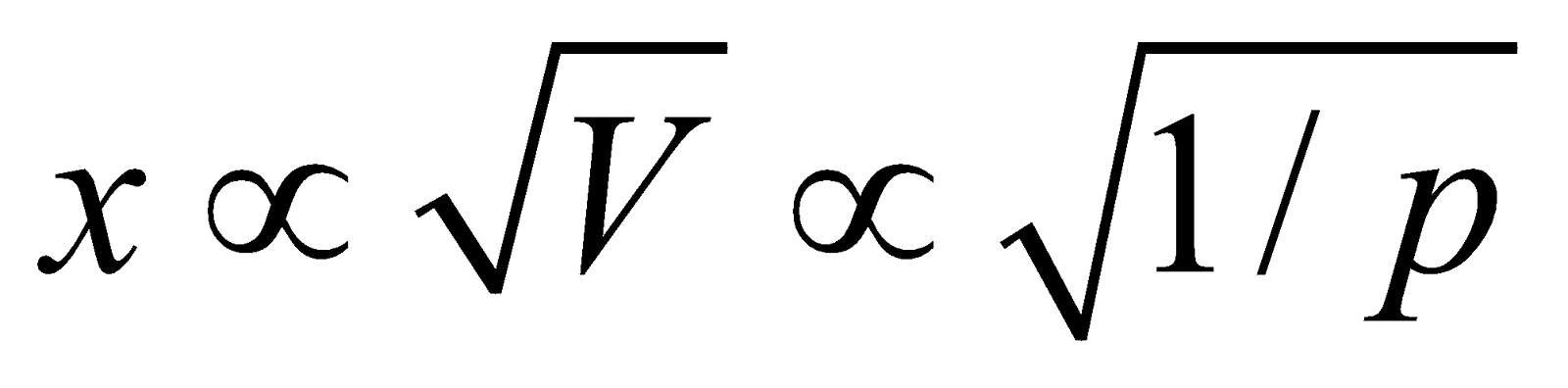
LIQUID SYSTEM
Examples are :
- Esterification of acetic acid
At equilibrium 2/3rd of acetic acid is converted into ester. Hence alcohol consumed will also be 2/3rd.
- Reaction between amylene and tricholoroacetic acid
CHEMICAL EQUILIBRIUM APPLIED TO HETEROGENEOUS SYSTEM
- Dissociation of calcium carbonate
Applying the law of chemical equilibrium
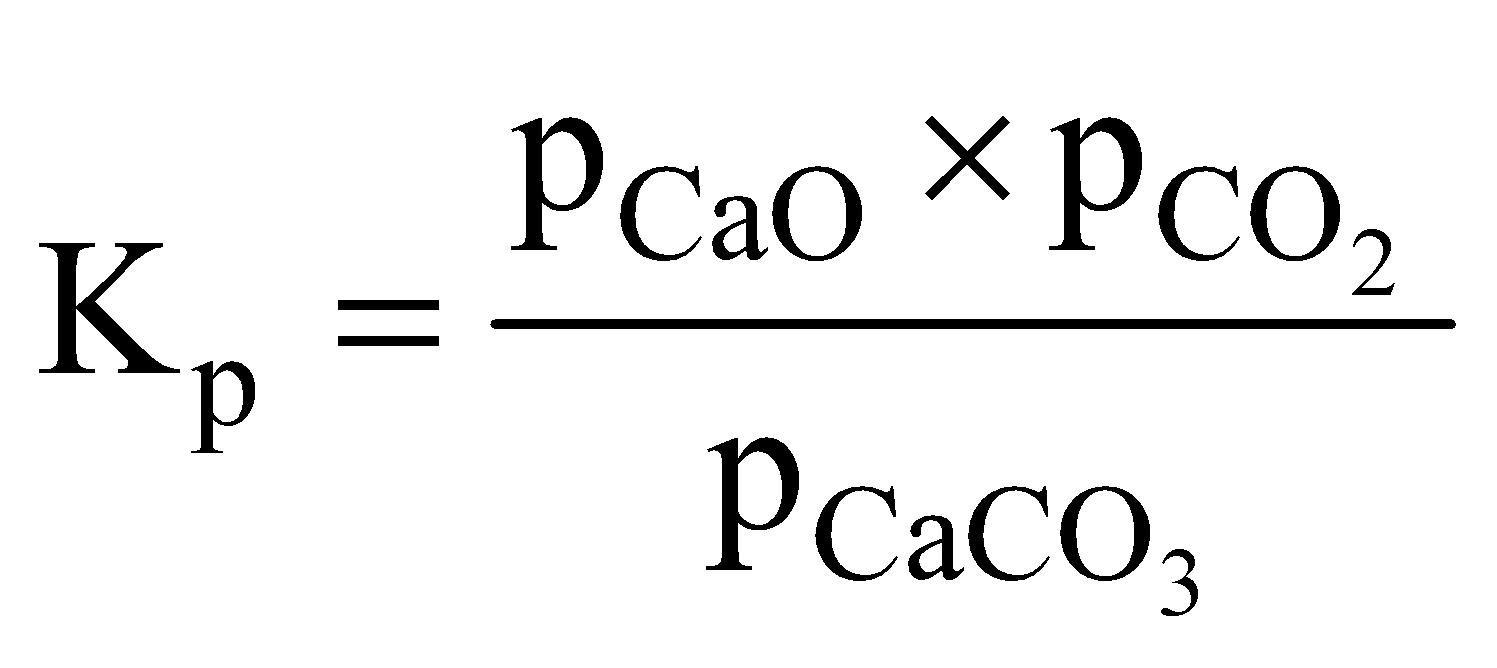
The active mass of a solid reactant and product is assumed to have a constant value and is taken as unity. The equilibrium constant is determined by gaseous substances only. Therefore, 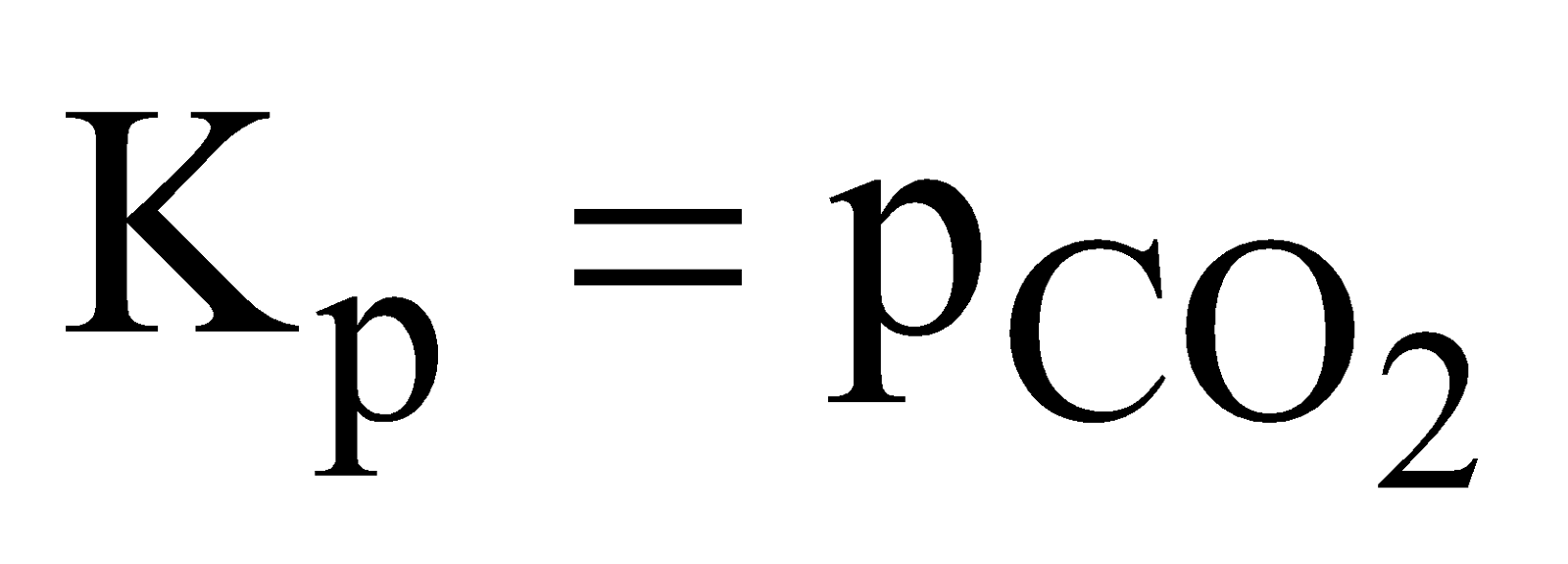
- Reaction of steam on heated iron
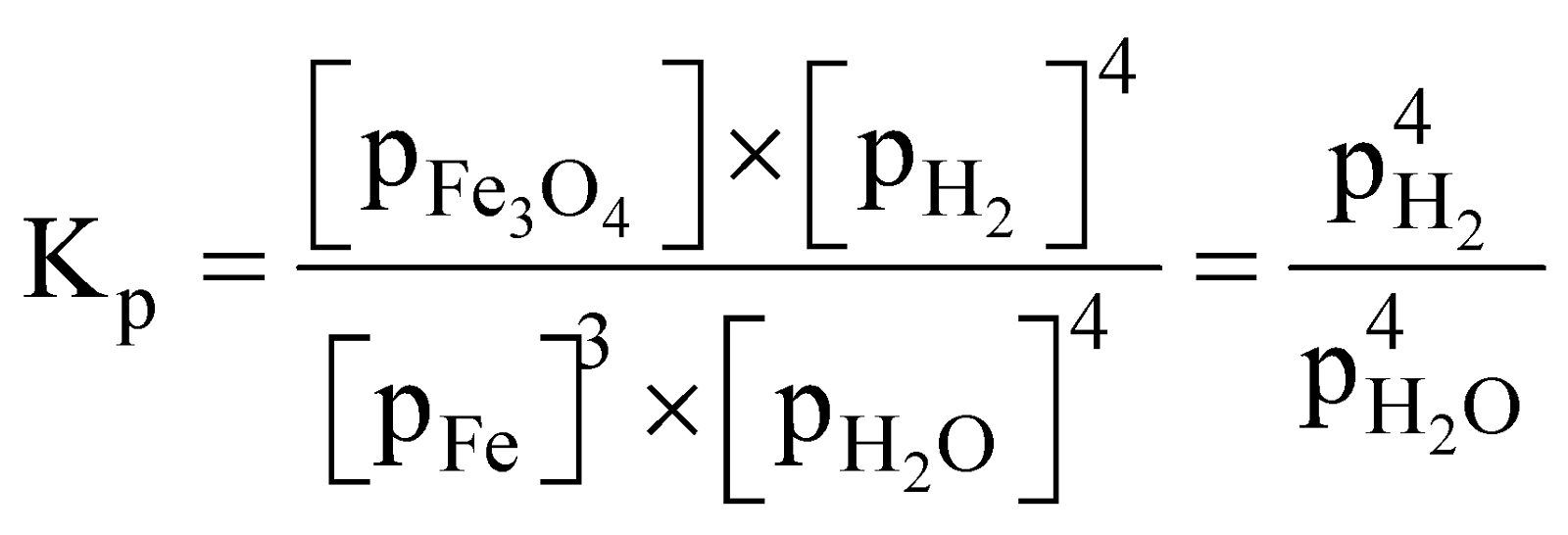
Partial pressures of solid is taken unity.
- Water gas reaction
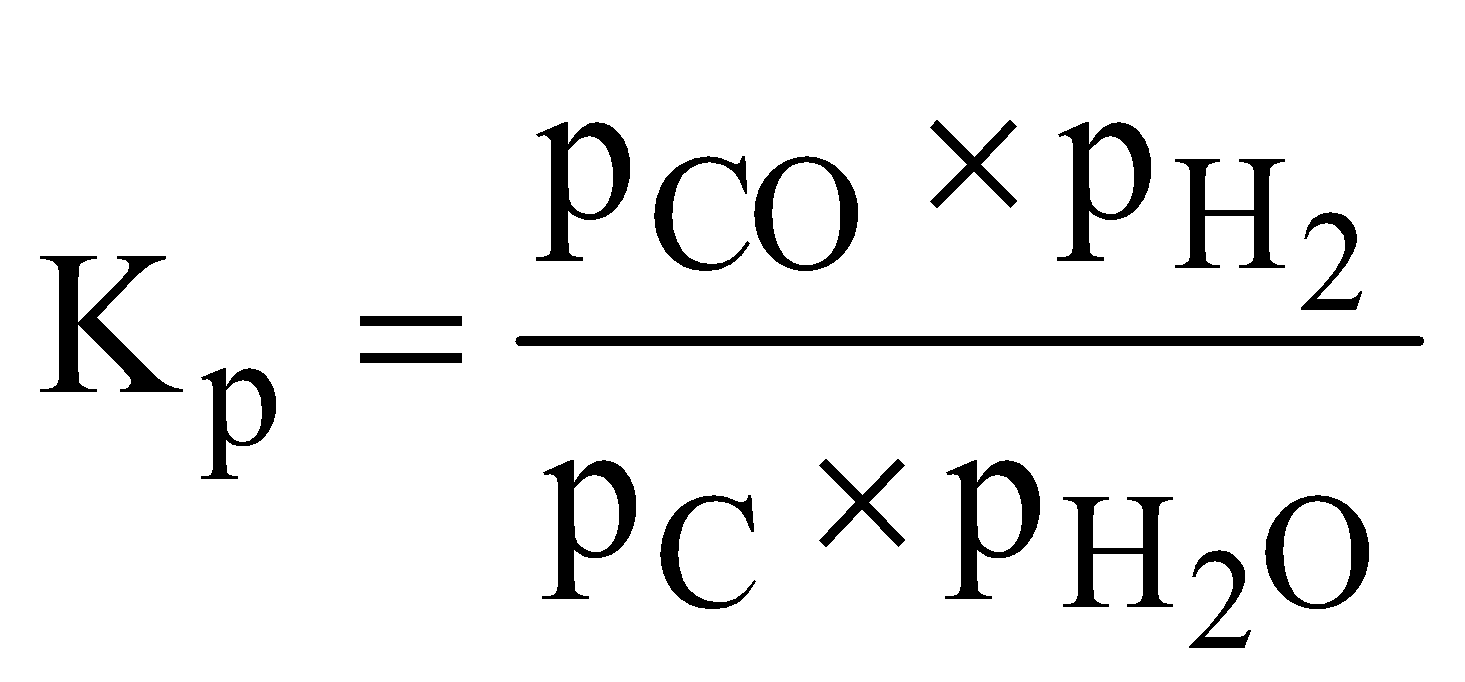
Since partial pressure of carbon (solid) is taken as unity, the equilibrium constant is given by
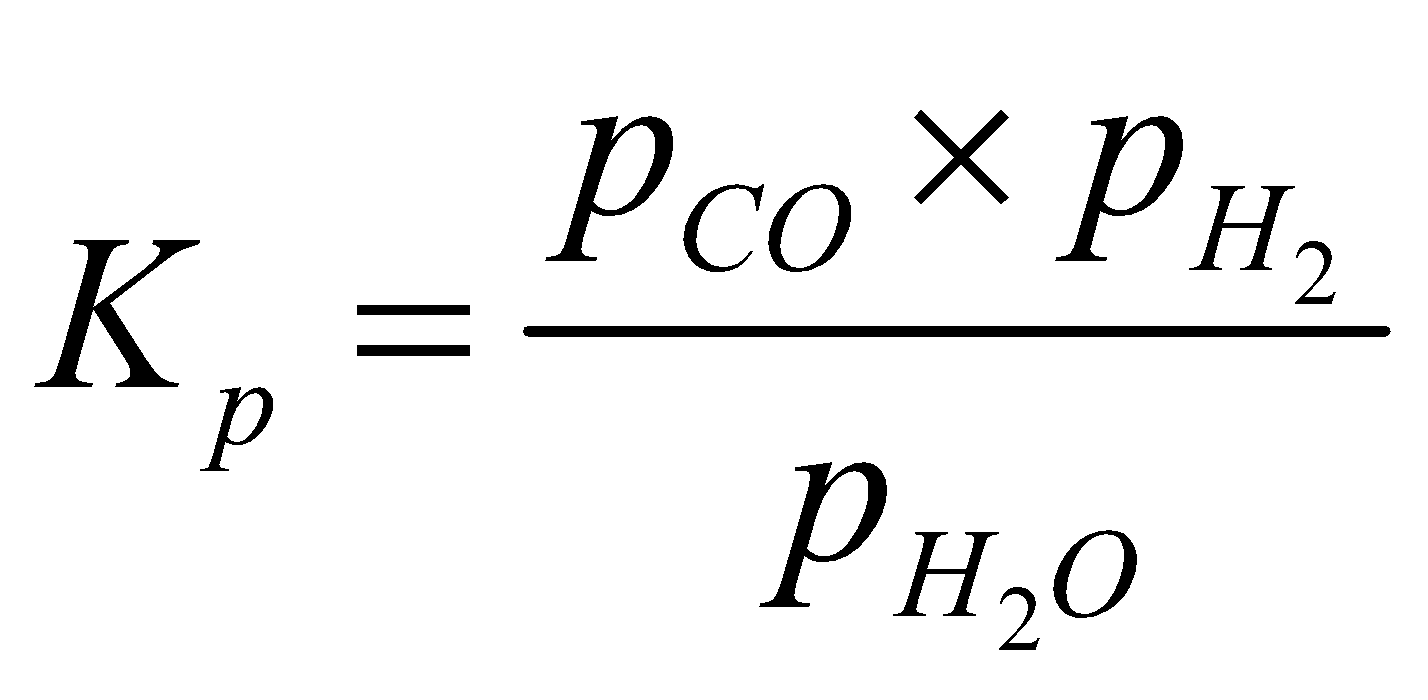
VAN’T HOFF ISOCHORE
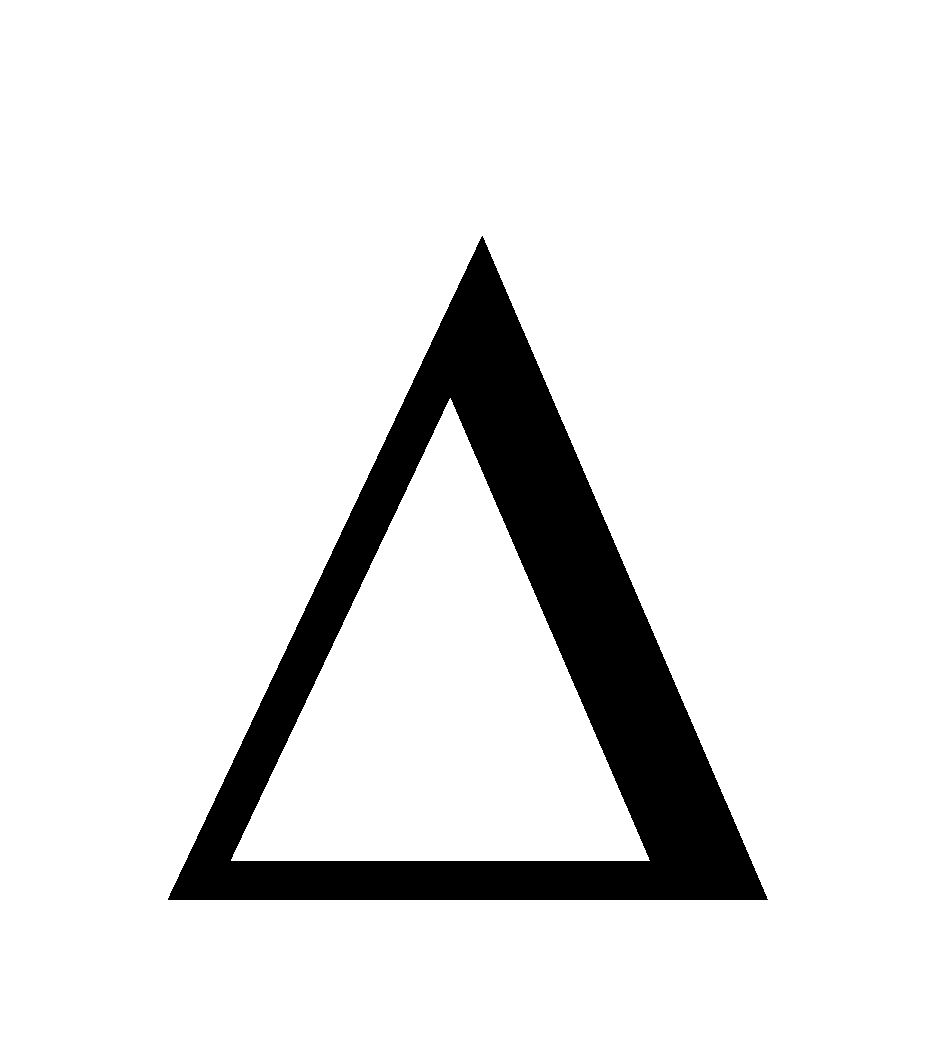
A relationship between the equilibrium constant Kp, at any temperature T and constant pressure P, and heat of reaction  H°.
H°.
The enthalpy change DH does not vary appreciably with change in partial pressures of reactants and products. Therefore DHº can be taken as DH whatever may be the partial pressures of reactants and products
The integrated form of the equation is
Three important conditions may arise
- when
 H = 0 no heat is evolved or absorbed
H = 0 no heat is evolved or absorbed
Equilibrium constant does not change when no change in temperature.
- when
 H = +ve i.e. heat is absorbed
H = +ve i.e. heat is absorbed
Equilibrium constant increases with increase of temperature.
- when
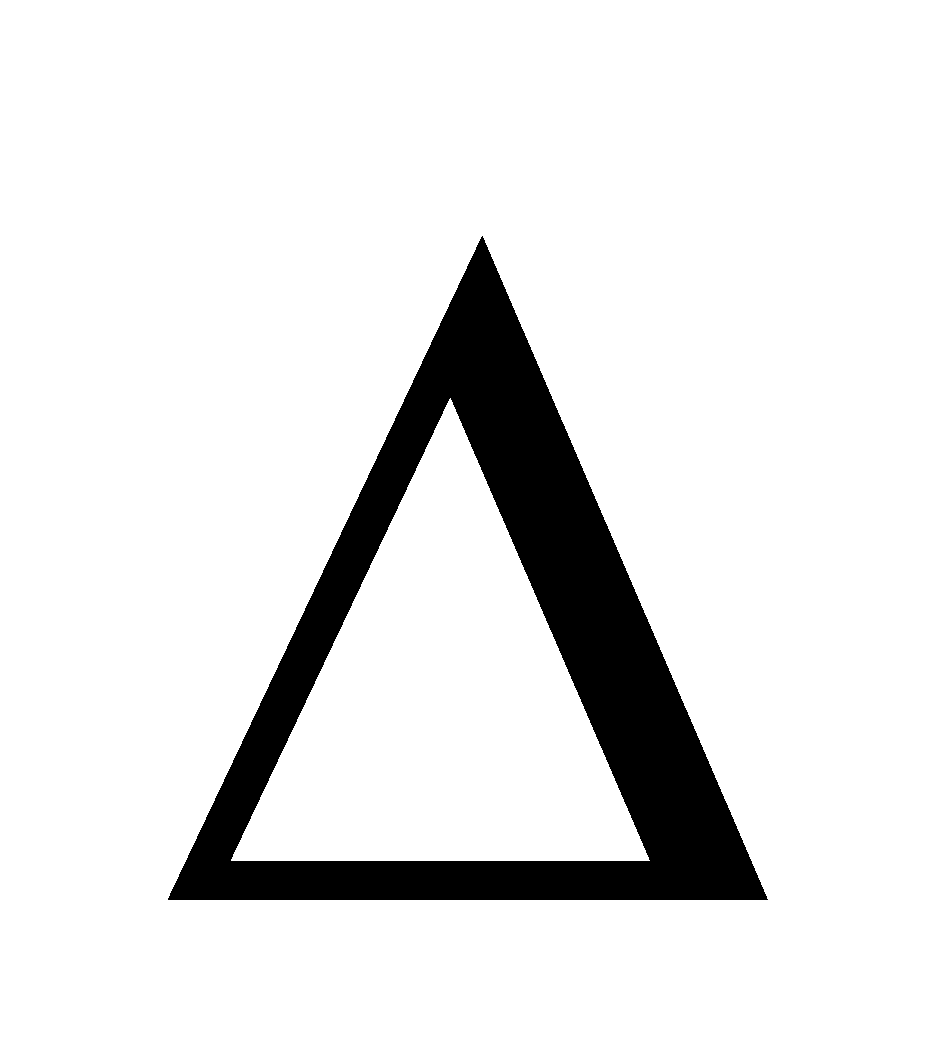 H = –ve i.e. heat is evolved
H = –ve i.e. heat is evolved
decreases with increase of temperature
- when
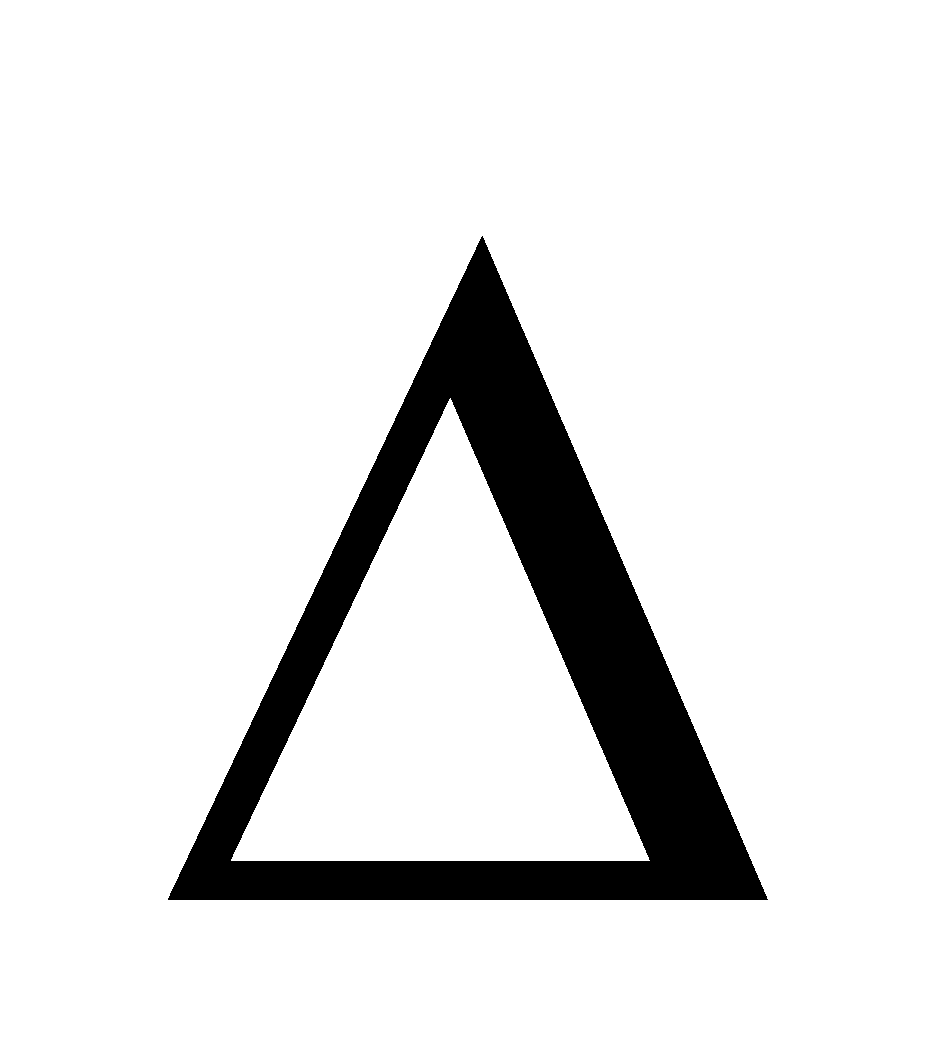 n = 0 i.e. there is no change in volume during a reaction KP = Kc. The variation of equilibrium constant with temperature is given by
n = 0 i.e. there is no change in volume during a reaction KP = Kc. The variation of equilibrium constant with temperature is given by
VAN’T HOFF REACTION ISOTHERM
It gives the free energy change of a reaction at any given temperature, pressure and composition of the reacting system.
At equilibrium 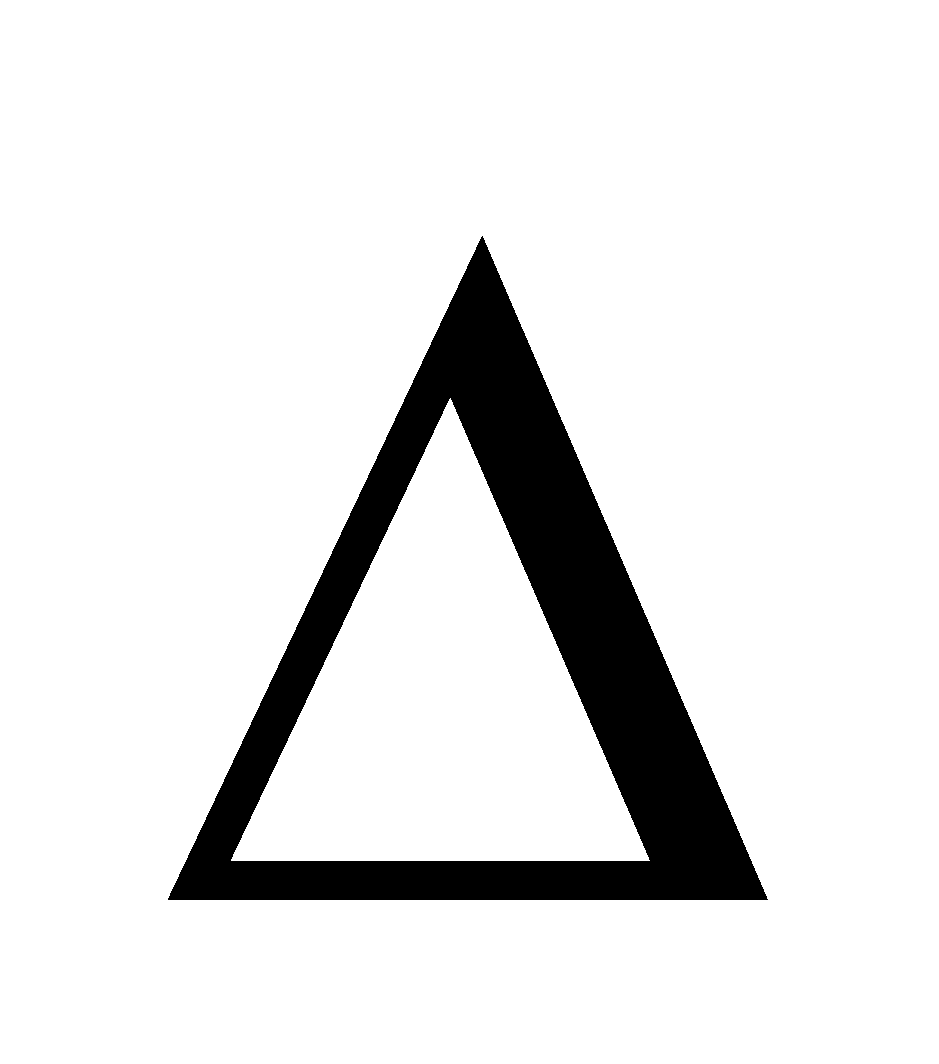 G = 0 then
G = 0 then 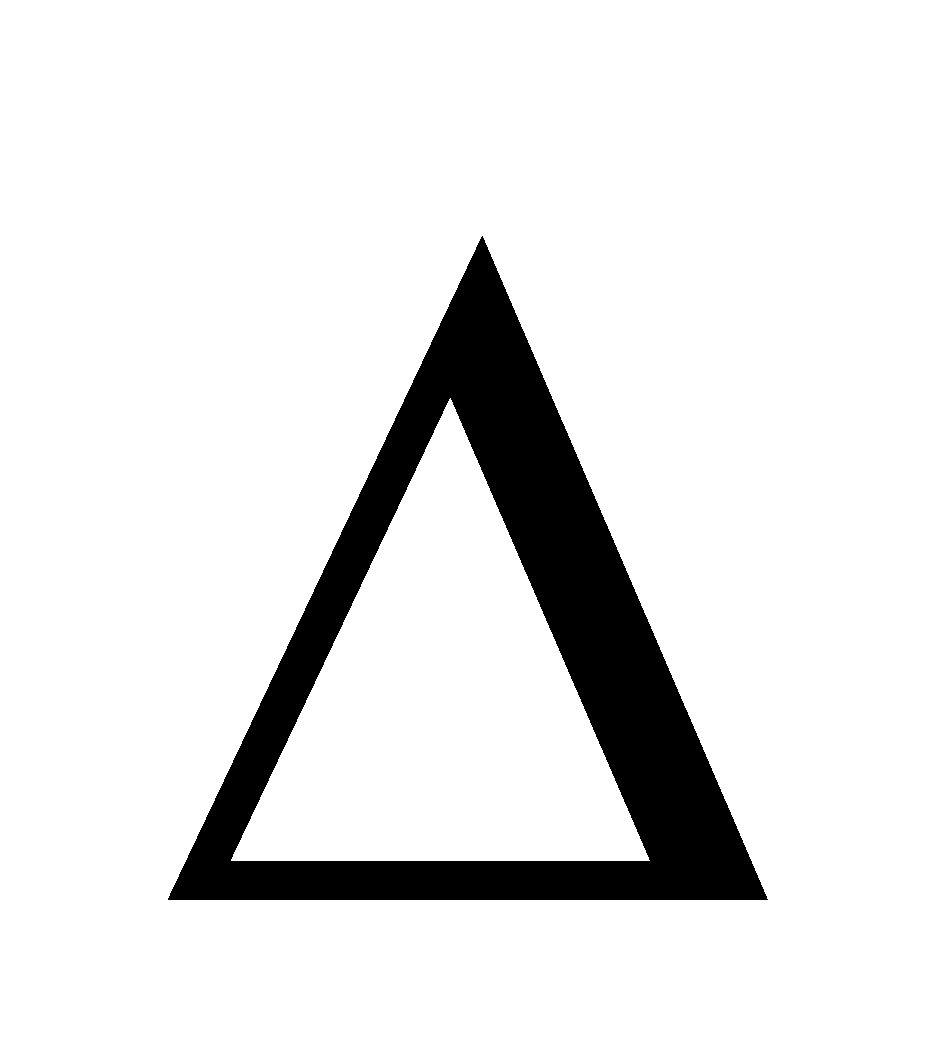 G° = –RT ln Jeq
G° = –RT ln Jeq
J stands for reaction quotient of partial pressure of products and reactants.
viz. 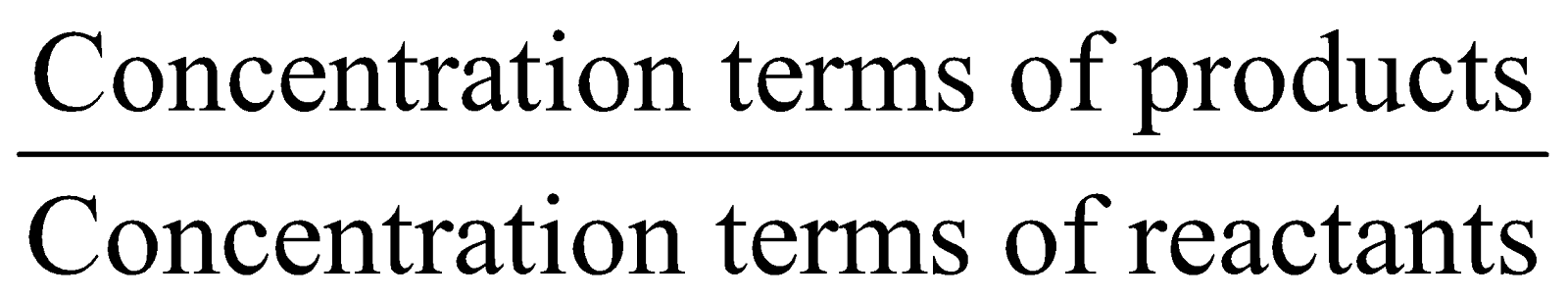
Jeq means the partial pressure of the products and the reactants at the equilibrium. Hence Jeq can be replaced by Kp.
HENRY’S LAW
The mass of a gas dissolved per unit volume of solvent is proportional to the pressure of the gas in equilibrium with the solution at constant temperature.
- The volume of the gas dissolved remains the same inspite of increase in pressure.
- The dissolution of a gas in a liquid is spontaneous process (
 G = 0), accompanied by decrease in entropy (
G = 0), accompanied by decrease in entropy ( S = –ve). Since
S = –ve). Since  G =
G = 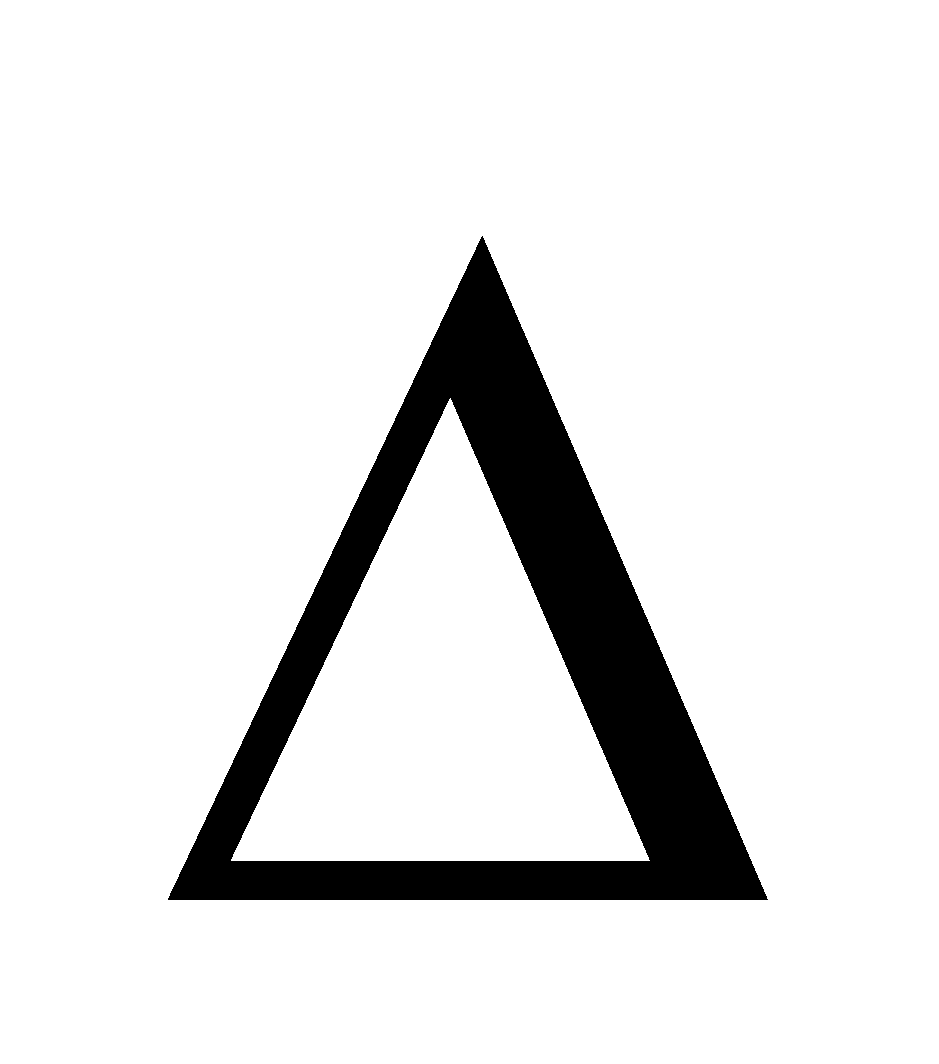 H – T
H – T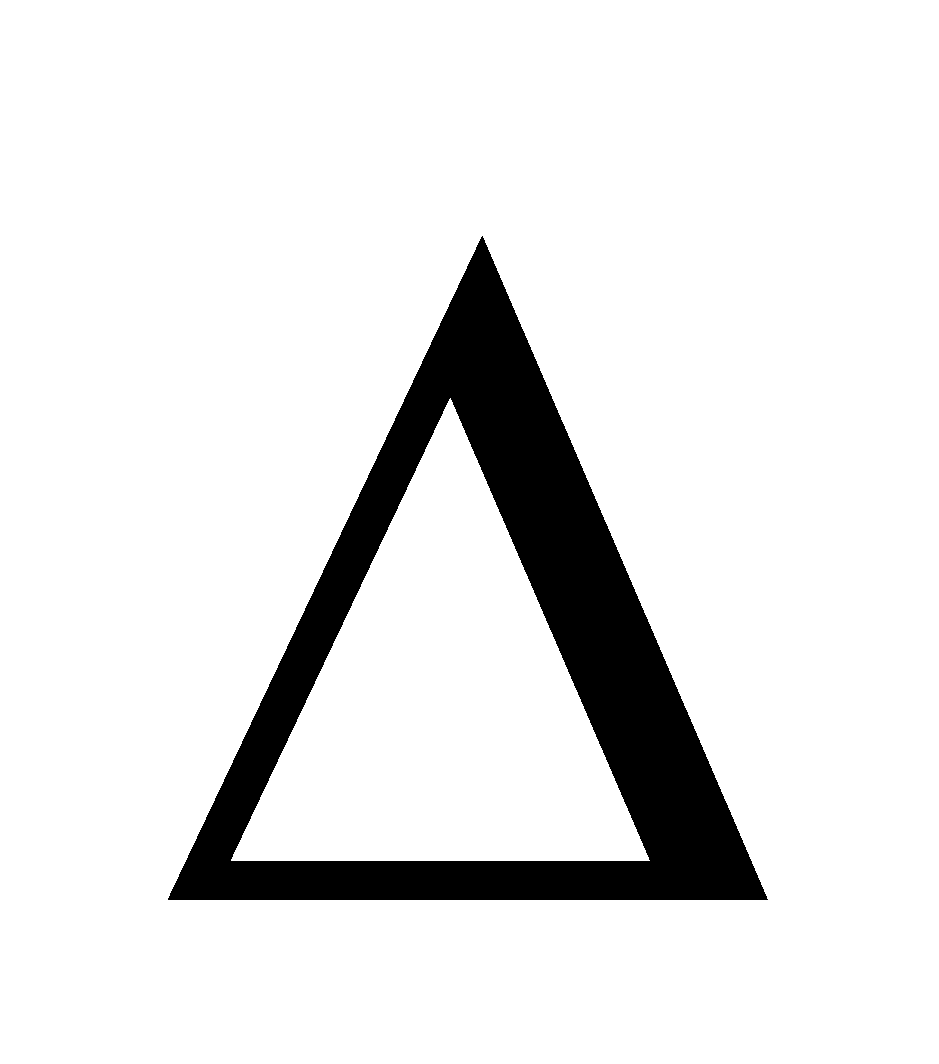 S,
S, 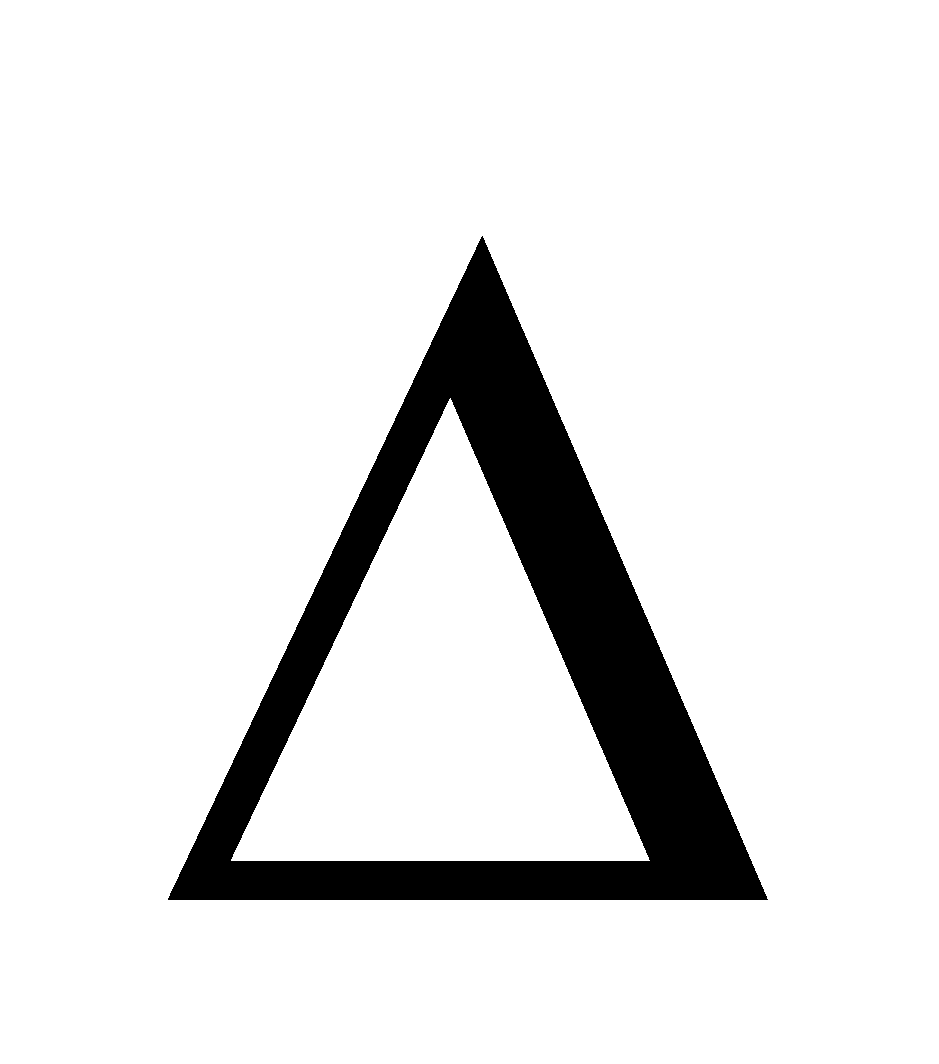 G can only be negative if
G can only be negative if 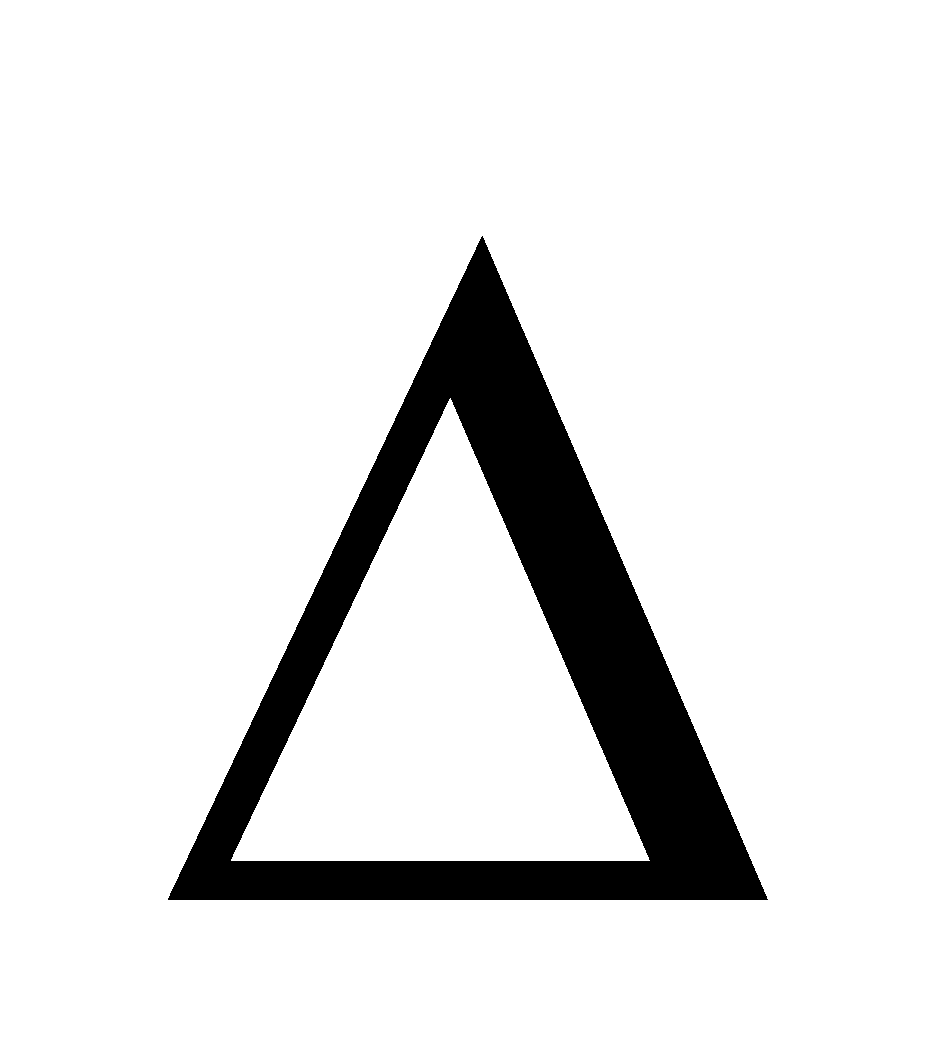 H is –ve. Therefore dissolution of a gas in a liquid is always exothermic in nature.
H is –ve. Therefore dissolution of a gas in a liquid is always exothermic in nature.
FACTORS ALTERING THE STATE OF EQUILIBRIUM – LE CHATELIER’S PRINCIPLE
There are three main factors which alter the state of equilibrium. They are (I) Concentration, (II) Temperature, and (III) Pressure.
Le Chatelier’s principle states that if a system at equilibrium is subjected to a change of concentration, pressure or temperature, the equilibrium shifts in the direction that tends to undo the effect of the change.
- Effect of change of concentration :
If at equilibrium the concentration of one of the reactants is increased, the equilibrium will shift in the forward direction and vice versa. Consider the following equilibrium
Fe3+ (aq) + SCN– (aq) 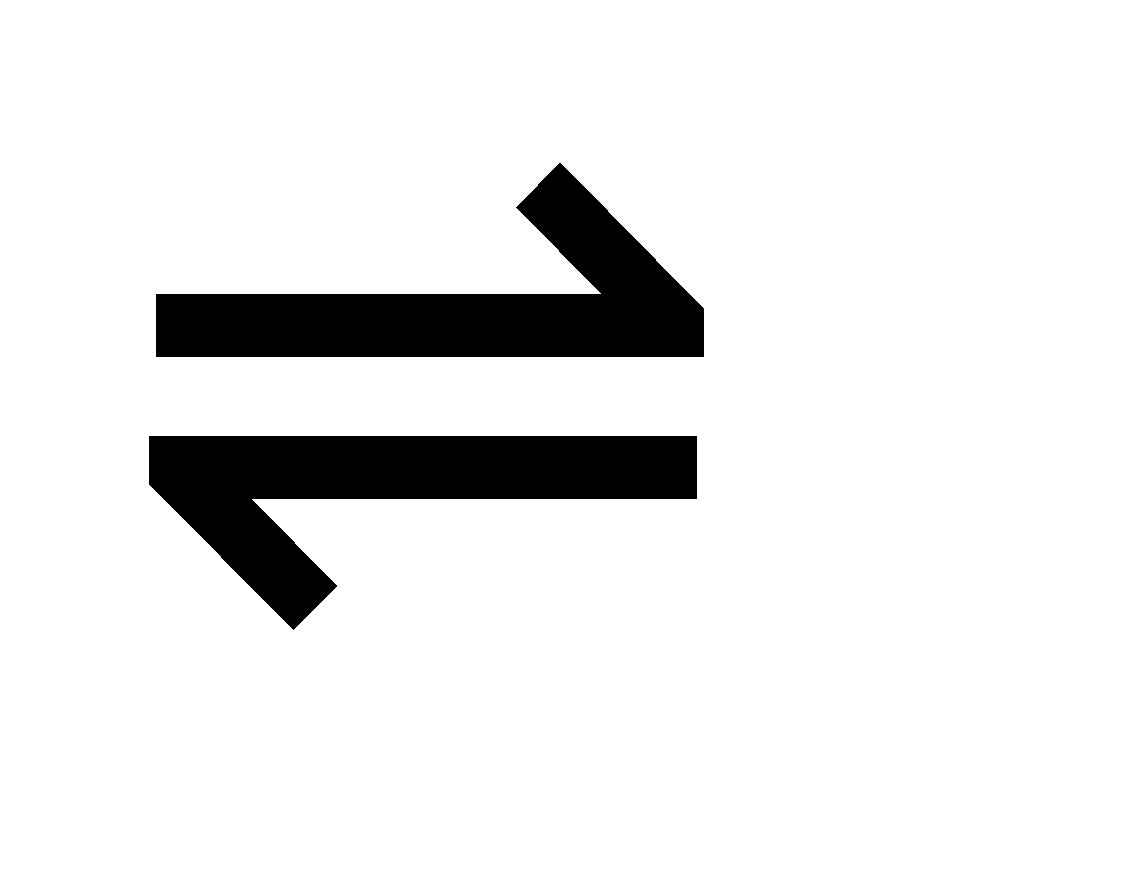 [Fe(SCN)]2+ (aq)
[Fe(SCN)]2+ (aq)
Pale yellow Colourless Dark brown
If ferric salt is added the colour of the solution darkens immediately i.e. Fe3+ ions are consumed and more [Fe(SCN)]2+ are formed. If some sulphocyanide salt is added the colour also darkens. If Potassium ferrisulphocyanide capable of giving complex ion [Fe(SCN)]2+ is added the colour lightens to pale yellow.
- Effect of change in pressure :
- No effect of pressure on equilibria having same moles of reactants and products e.g. N2 + O2
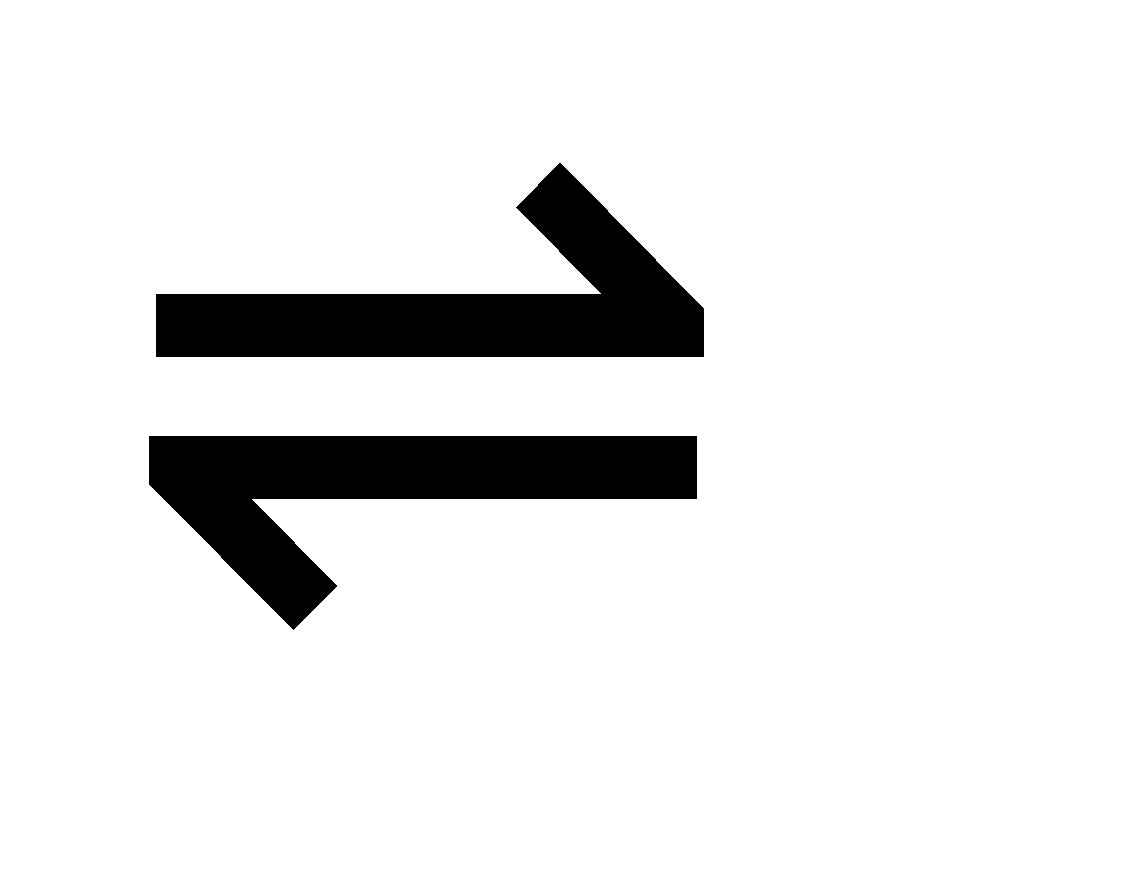 2NO
2NO
H2 + I2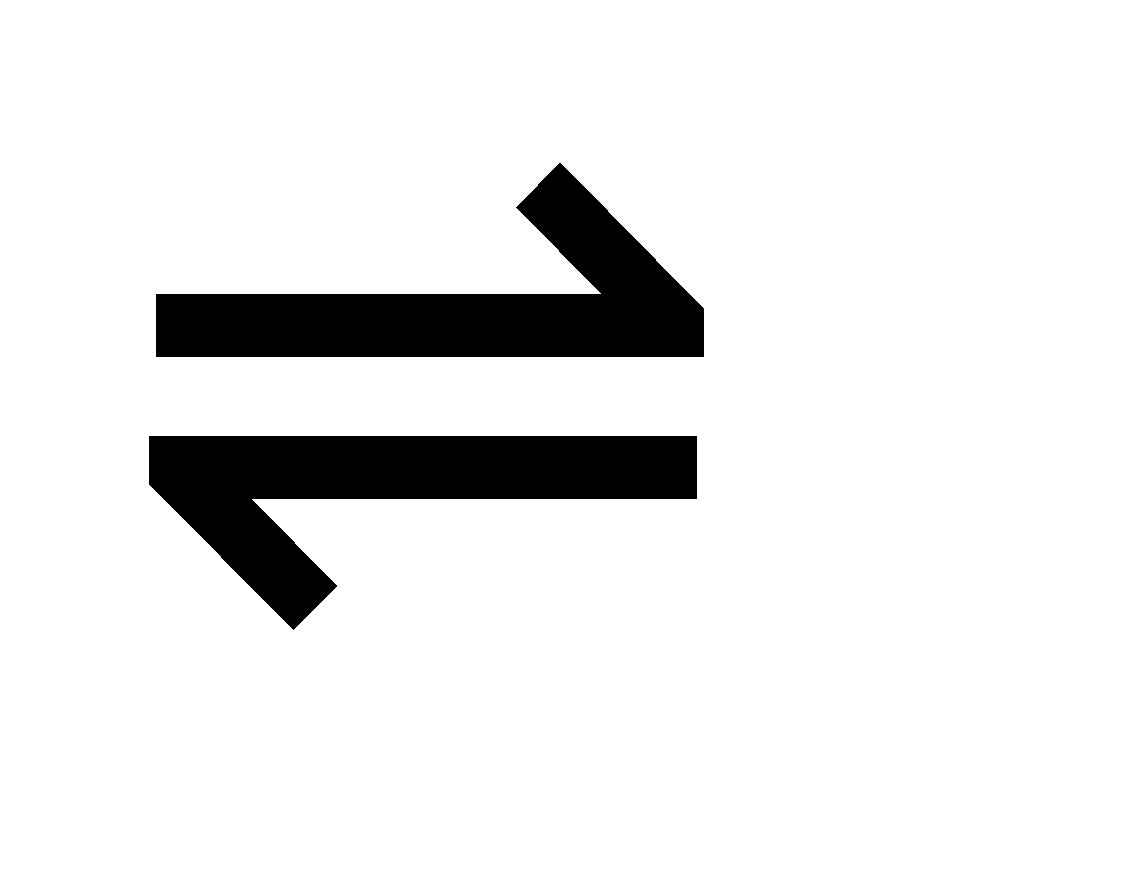 2HI
2HI - When there is change in the number of moles the equilibrium will shift in the direction having smaller number of moles when the pressure is increased and vice versa e.g.
N2 + 3H2 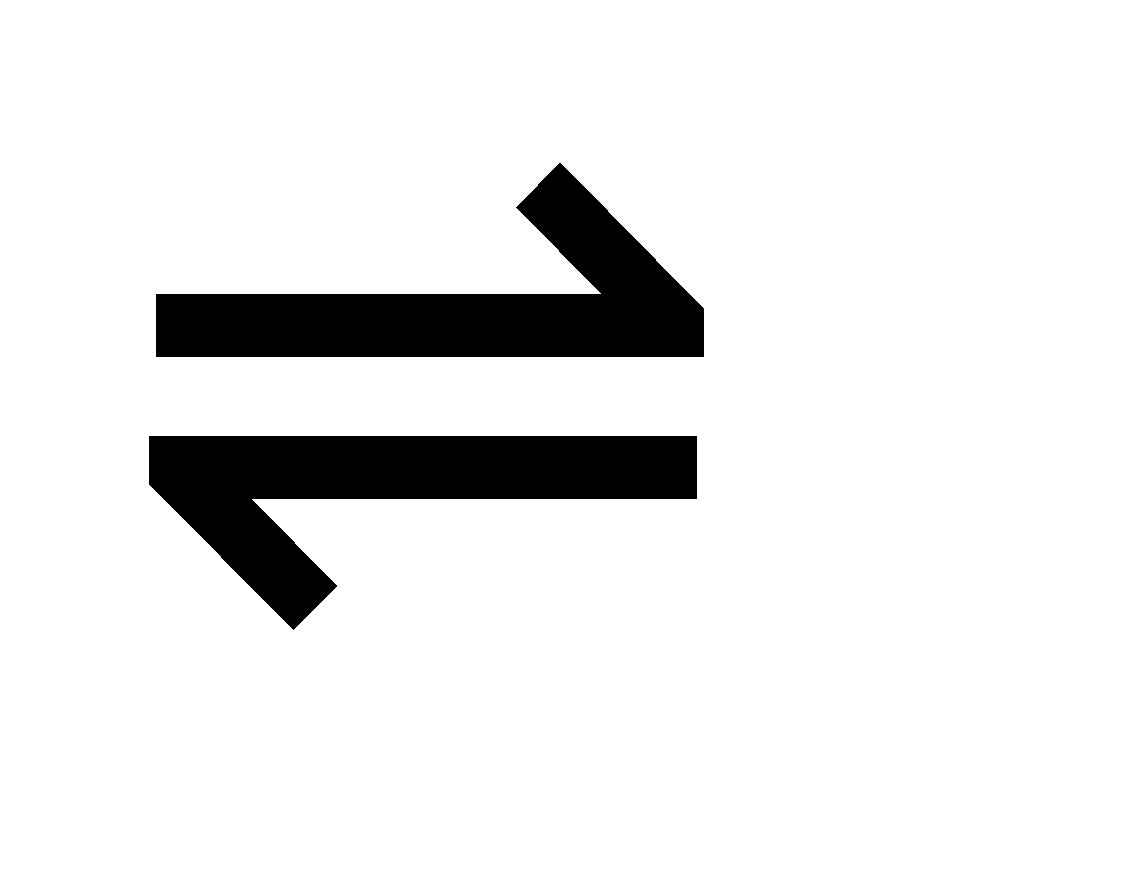 2NH3
2NH3
More pressure more ammonia
PCl5 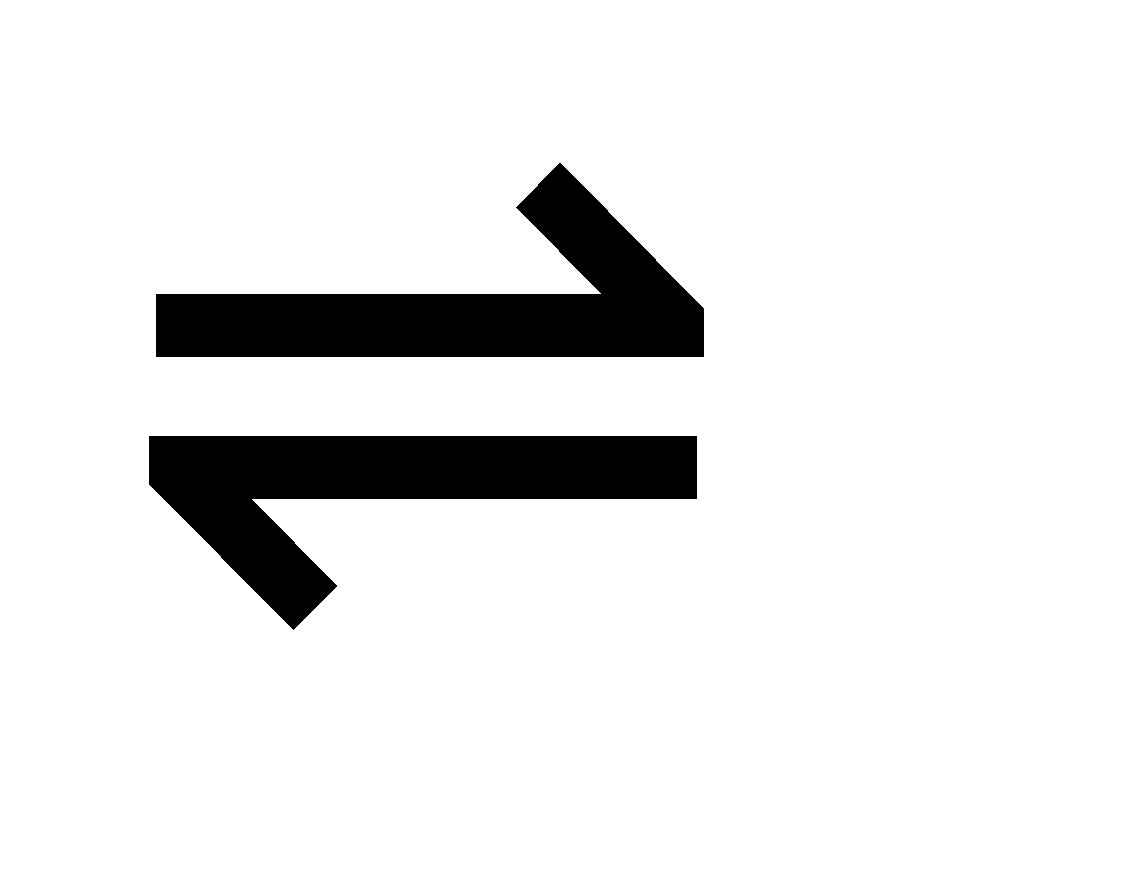 PCl3 + Cl2
PCl3 + Cl2
The more the pressure, the lesser the dissociation of PCl5.
- Effect of temperature :
- When process is exothermic – Low temperature favours the formation of products.
- When process is endothermic – High temperature favours the formation of products
e.g. N2 + 3H2 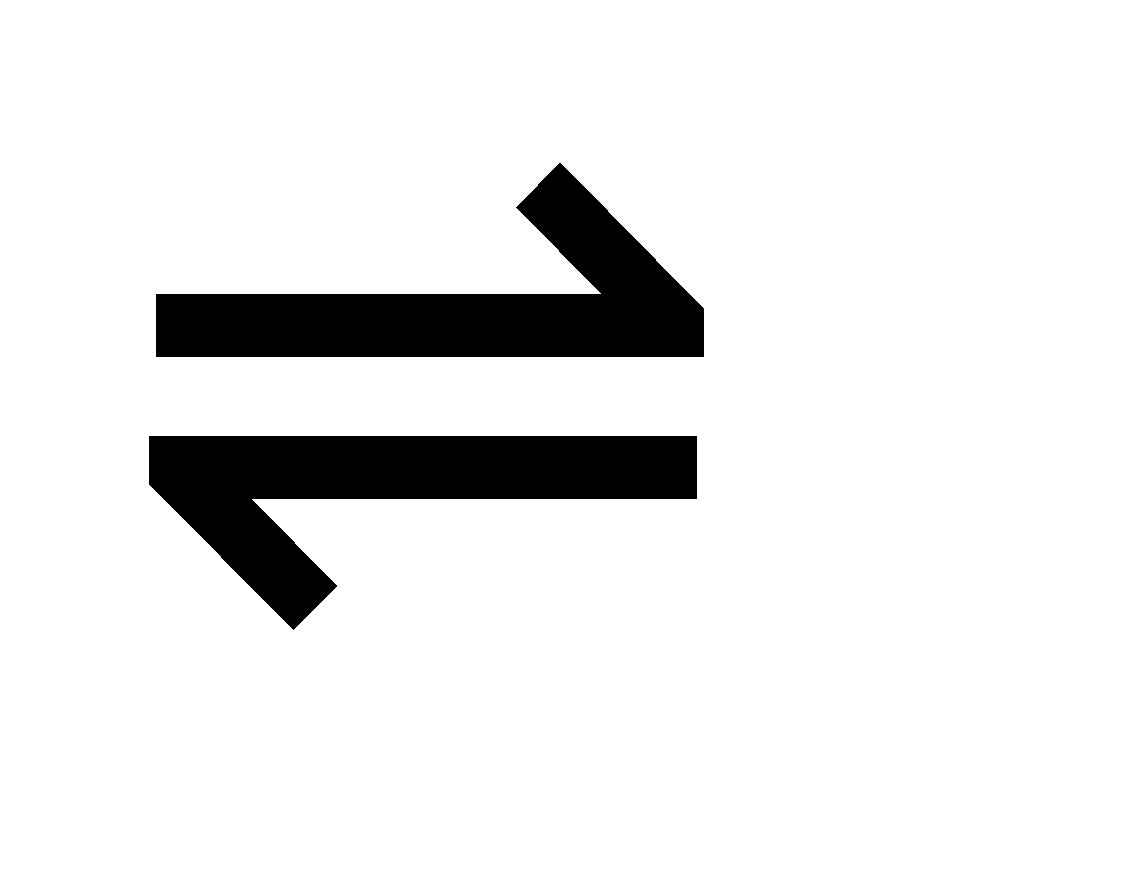 2NH3 + 24.0 kcal.
2NH3 + 24.0 kcal.
Since the production of NH3 is exothermic low temperature favours its formation.
- Effect of addition of inert gas :
- Addition of Inert gas at constant volume : The total pressure of the system is increased, but the partial pressure of each reactant and product remains the same. Hence no effect on the state of equilibrium.
- Addition of Inert gas at constant pressure : The total volume is increased, the number of moles per unit volume of each reactant and product is decreased. Hence equilibrium will shift to the side where number of moles are increased e.g.
PCl5 (g) 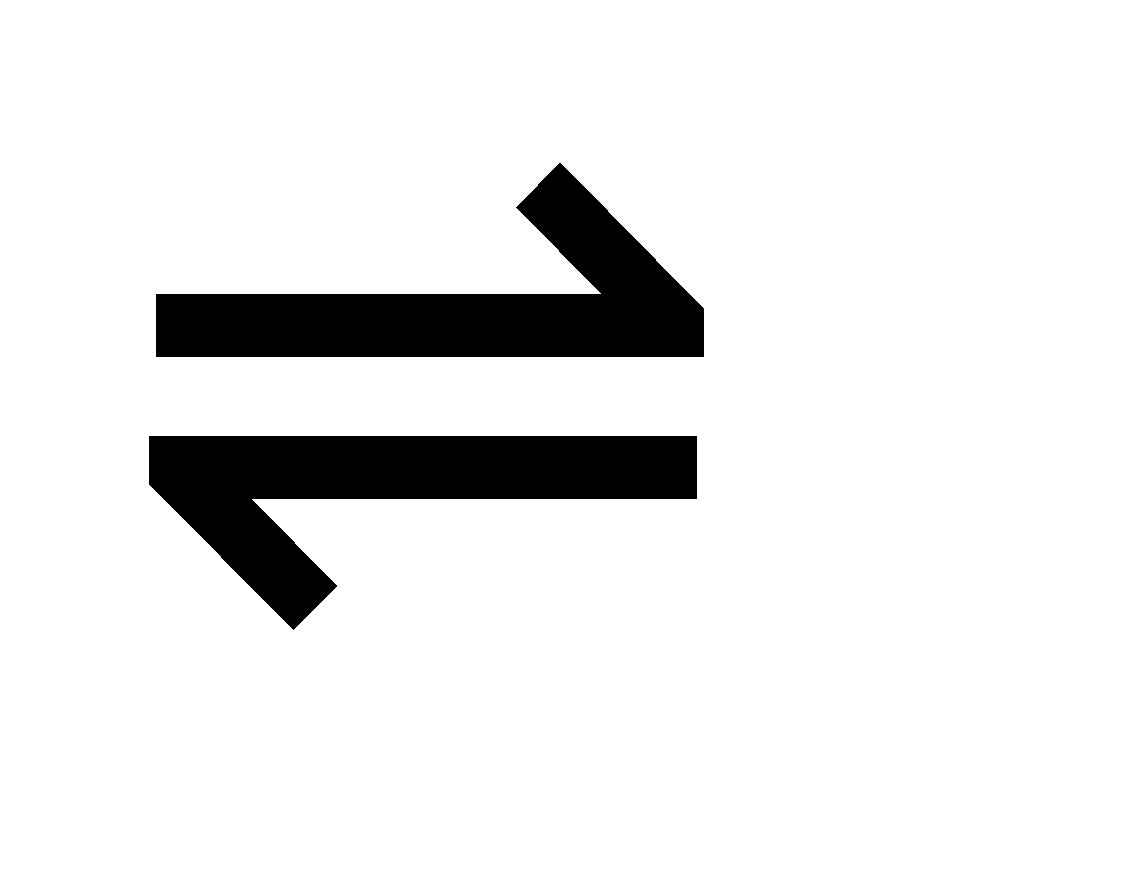 PCl3 (g) + Cl2 (g)
PCl3 (g) + Cl2 (g)
Introduction of inert gas at constant pressure will shift the equilibrium to right hand side.
- Effect of catalyst : The presence of catalyst does not change the position of equilibrium. It simply fastens the attainment of equilibrium.
LE CHATELIER’S PRINCIPLE APPLICABLE TO PHYSICAL EQUILIBRIUM
- Effect of pressure on solubility : The increased pressure, will increase the solubility of a gas and vice versa.
- Effect of temperature on solubility : The substances which dissolve with the absorption of heat, their solubility will increase with increase of temperature and vice versa e.g. dissolution of NH4Cl, KCl, KNO3 is endothermic which increases with increase of temperature. The dissolution of calcium acetate and Calcium hydroxide is exothermic, their solubility is lowered at higher temperature.
- Effect of pressure on the melting point of ice :
Ice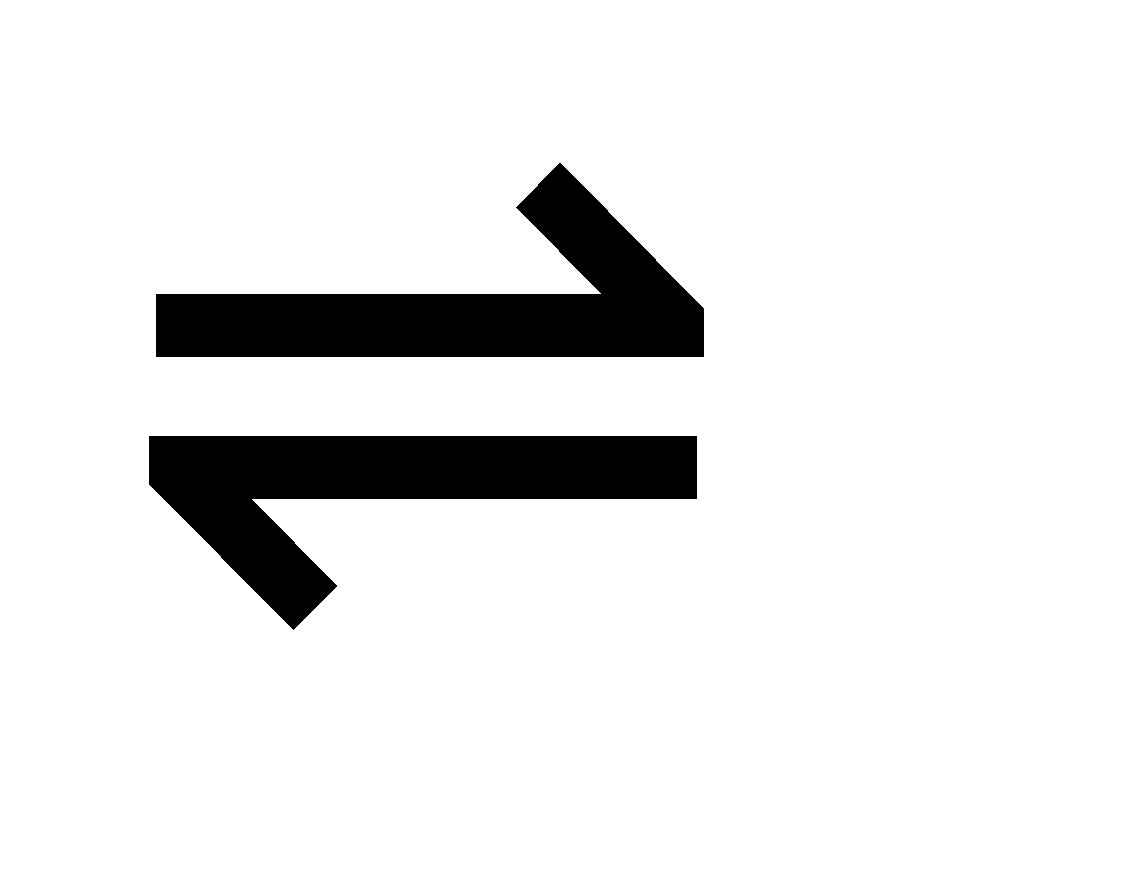 liquid water
liquid water
The ice occupy the more volume than liquid water, so increased pressure will result in melting of ice according to Le Chatelier’s principle.
FAVOURABLE CONDITIONS FOR SOME IMPORTANT REACTIONS
Synthesis of ammonia (Haber’s process)
N2 (g) + 3H2 (g) 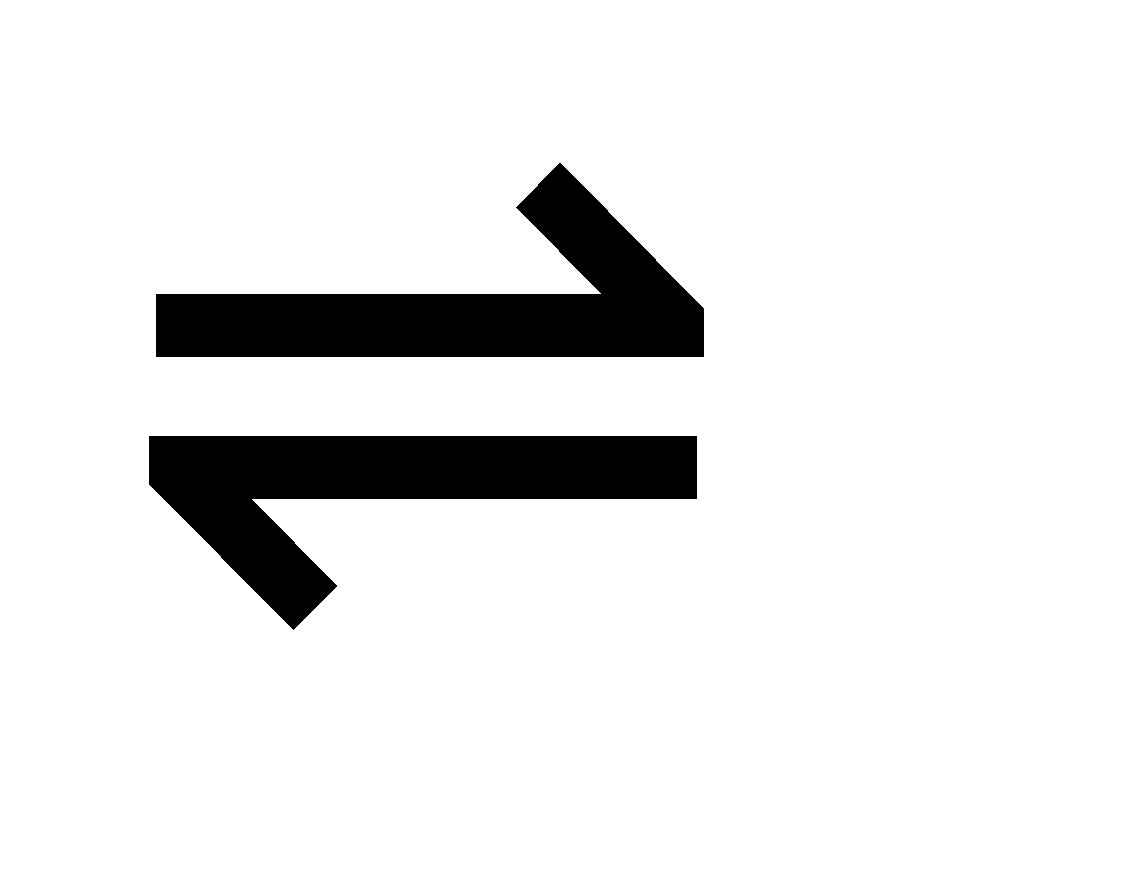 2NH3 (g) + 22.4 kcal
2NH3 (g) + 22.4 kcal
- Low temperature (500°C)
- High pressure (200 – 1000 atm.)
- Excess of N2 and H2
Synthesis of NO (nitric acid birkland eyde process)
N2 (g) + O2 (g) 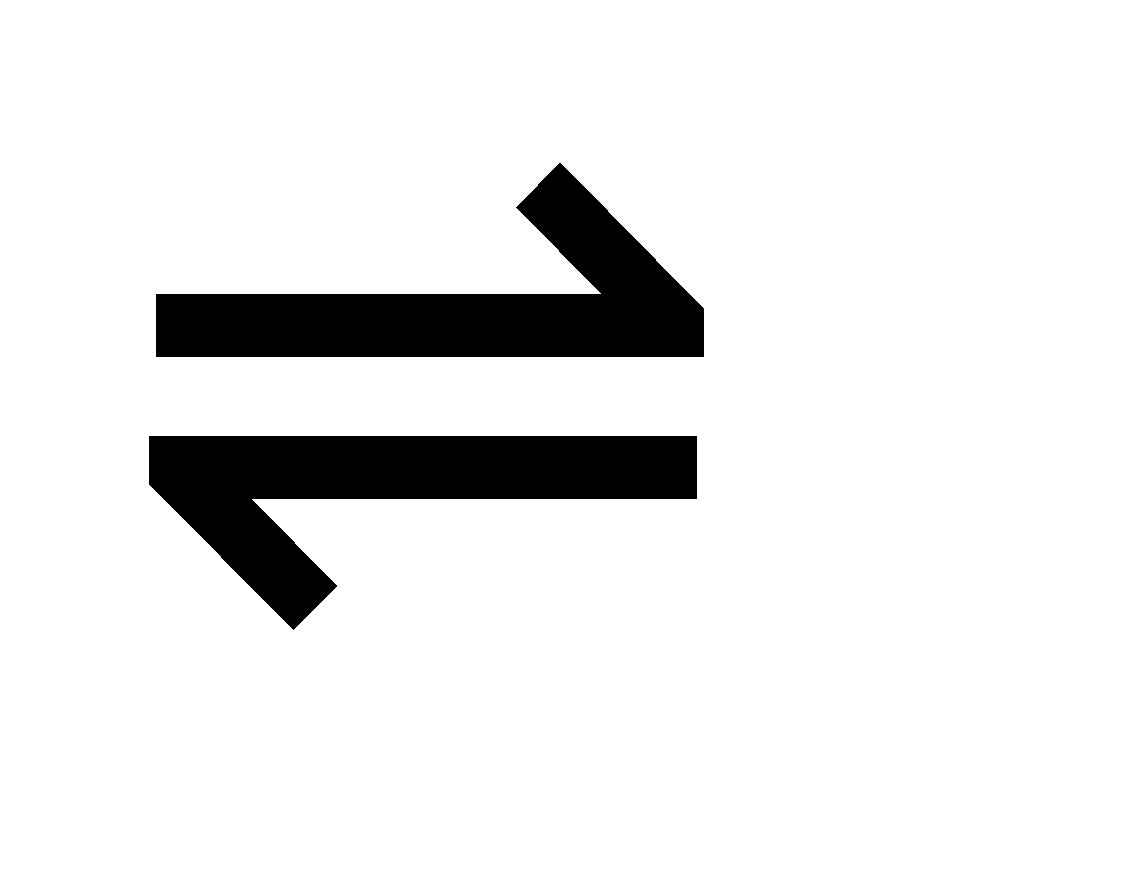 2NO(g)– 43.2 kcal
2NO(g)– 43.2 kcal
- High temperature
- Excess of N2 and O2
- No effect of pressure
Formation of SO3 (sulphuric acid contact process)
2SO2 (g) + O2 (g) 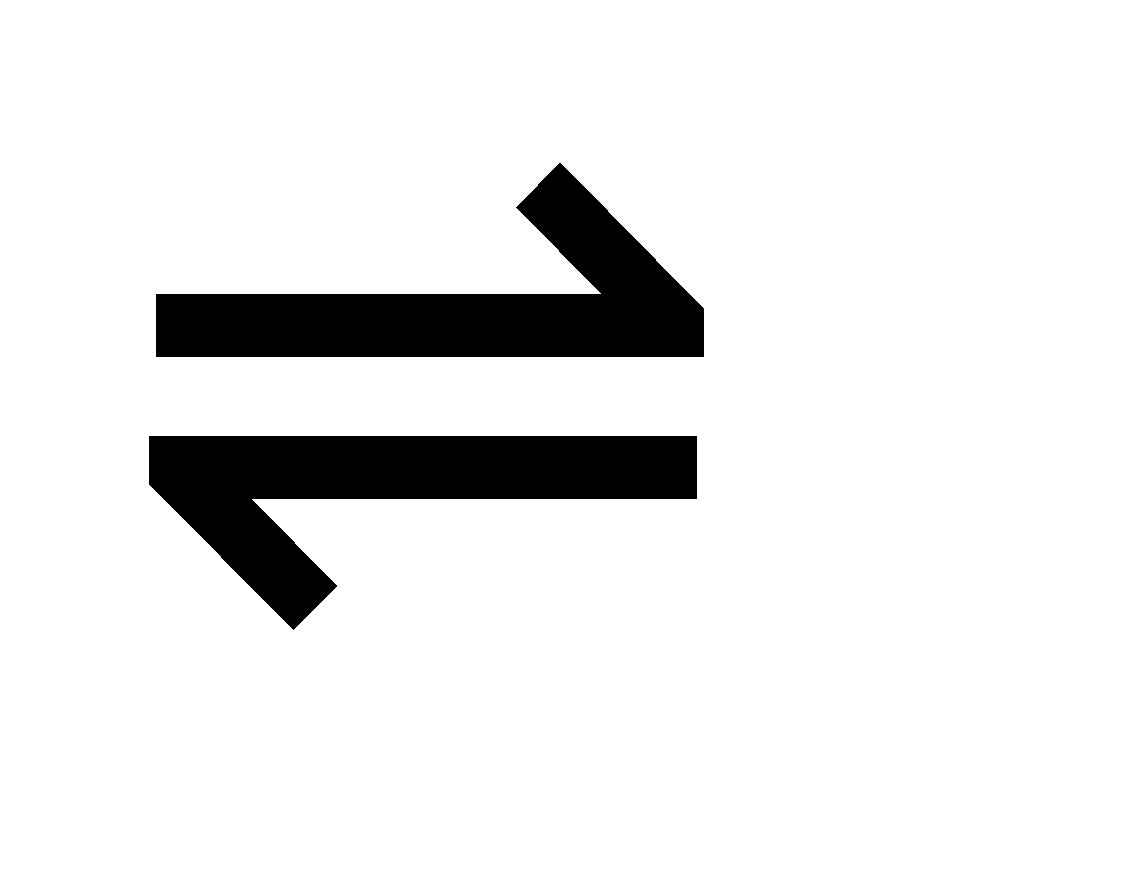 2SO3 + 42.0 kcal
2SO3 + 42.0 kcal
- Low temperature
- High pressure
- Excess of SO2 and O2
Formation of nitrogen dioxide
2NO + O2 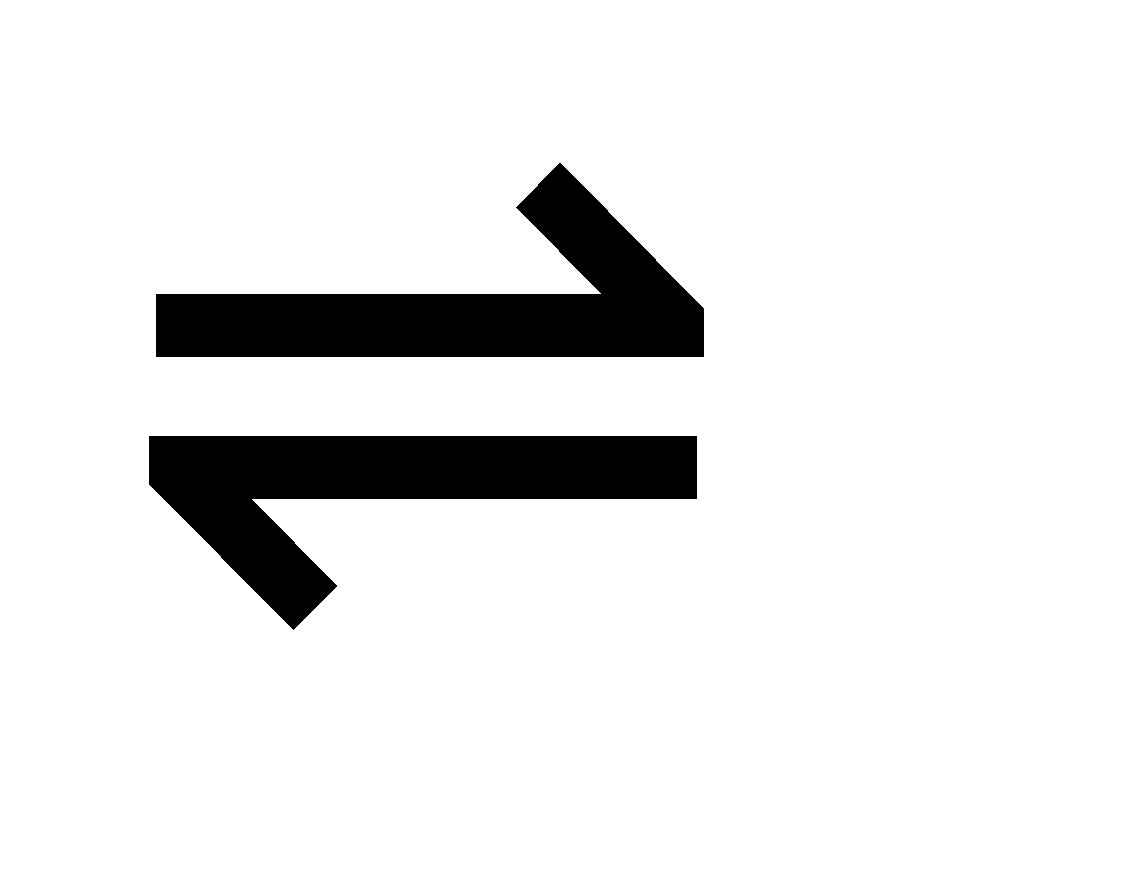 2NO2 + 27.8 kcal
2NO2 + 27.8 kcal
- Low temperature
- High pressure
- Excess of NO and O2
Dissociation of nitrogen tetraoxide
N2O4 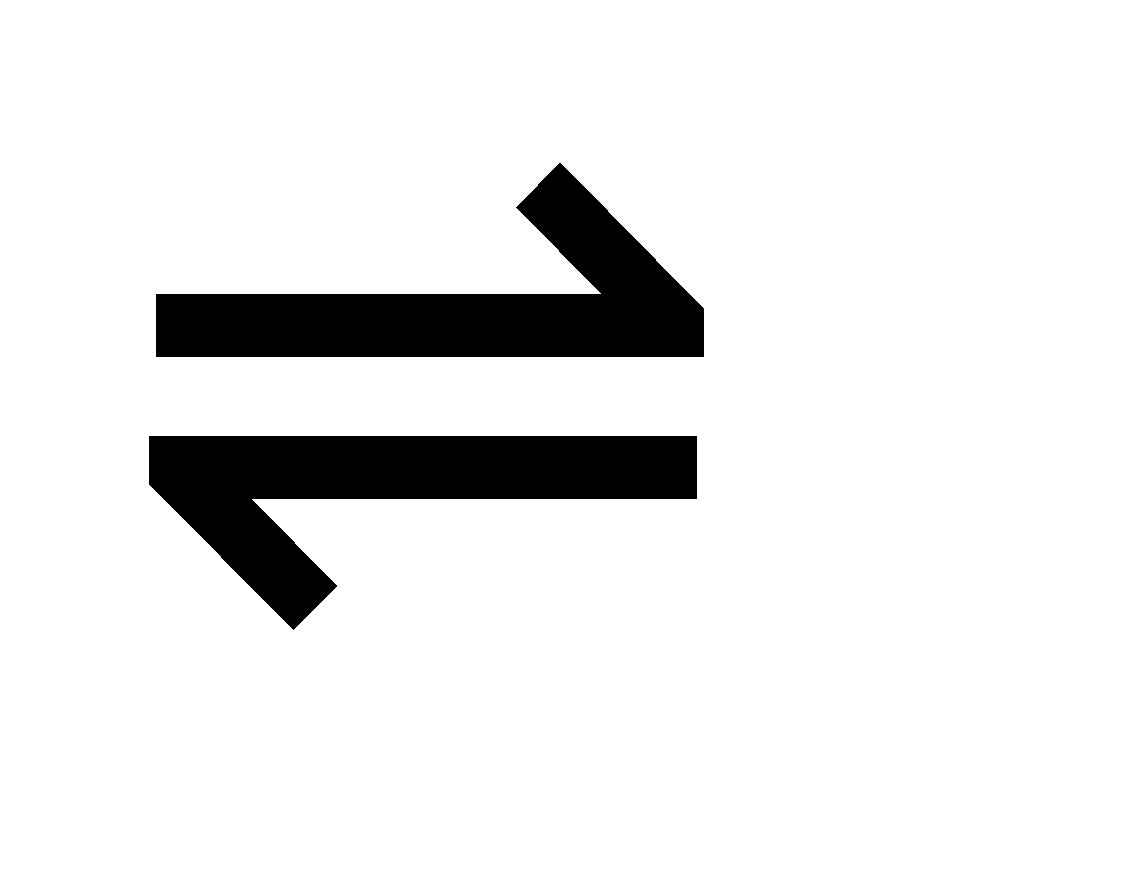 2NO2 – 14 kcal
2NO2 – 14 kcal
- High temperature
- Low pressure
- Excess of N2O4
Oxidation of CO by steam (Bosch process)
CO + H2O 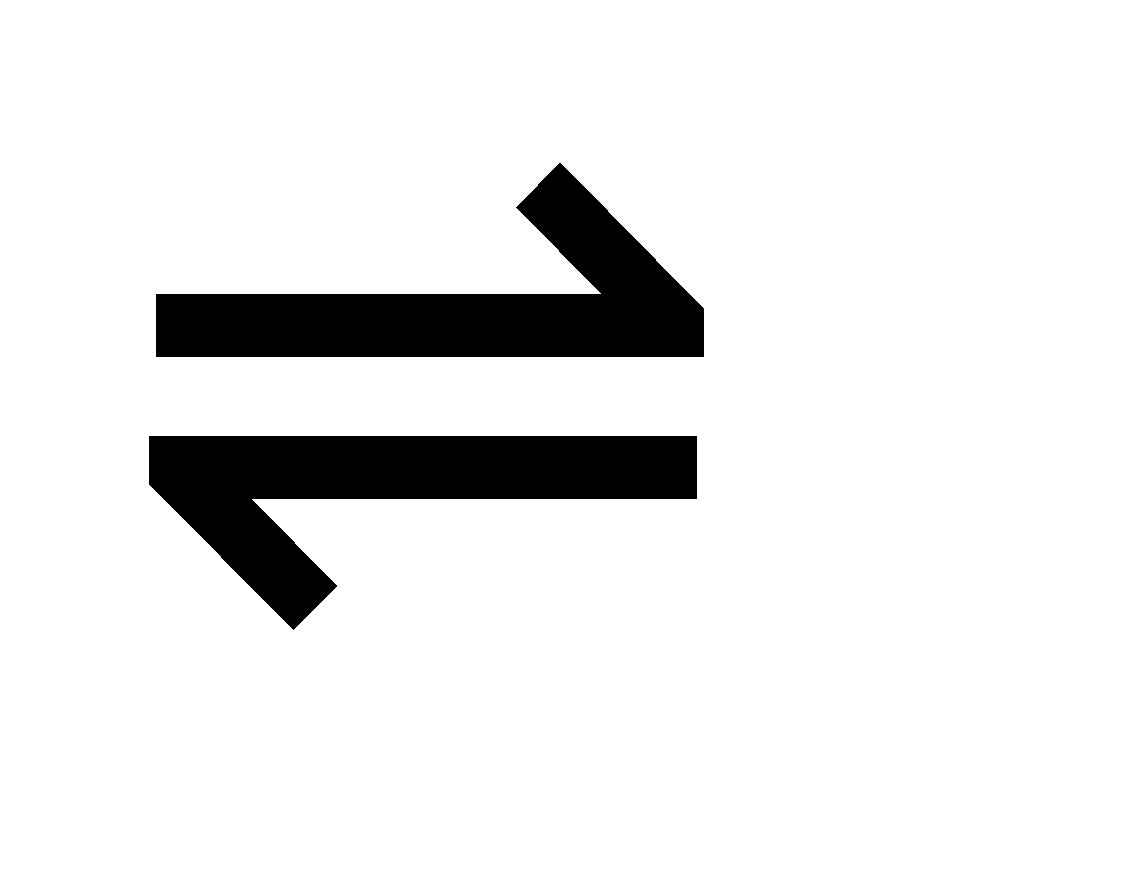 CO2 + H2 + x kcal
CO2 + H2 + x kcal
- Low temperature
- Excess of steam and CO
- No effect of pressure
Dissociation of PCl5
PCl5 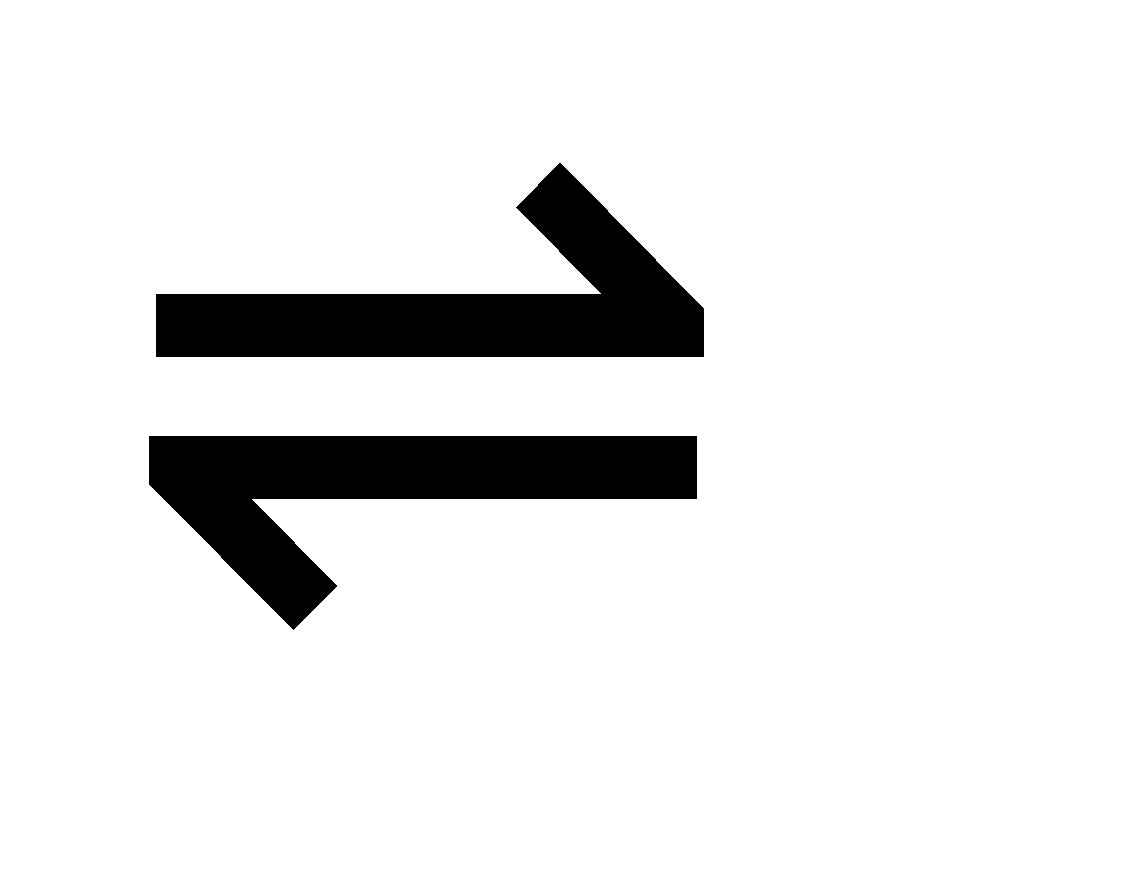 PCl3 + Cl2 – 15 kcal
PCl3 + Cl2 – 15 kcal
- High temperature
- Low pressure
- Excess of PCl5
TRIPLE POINT
The temperature and pressure at which the three states of a substance can exist in equilibrium is known as triple point e.g.
Ice (s) 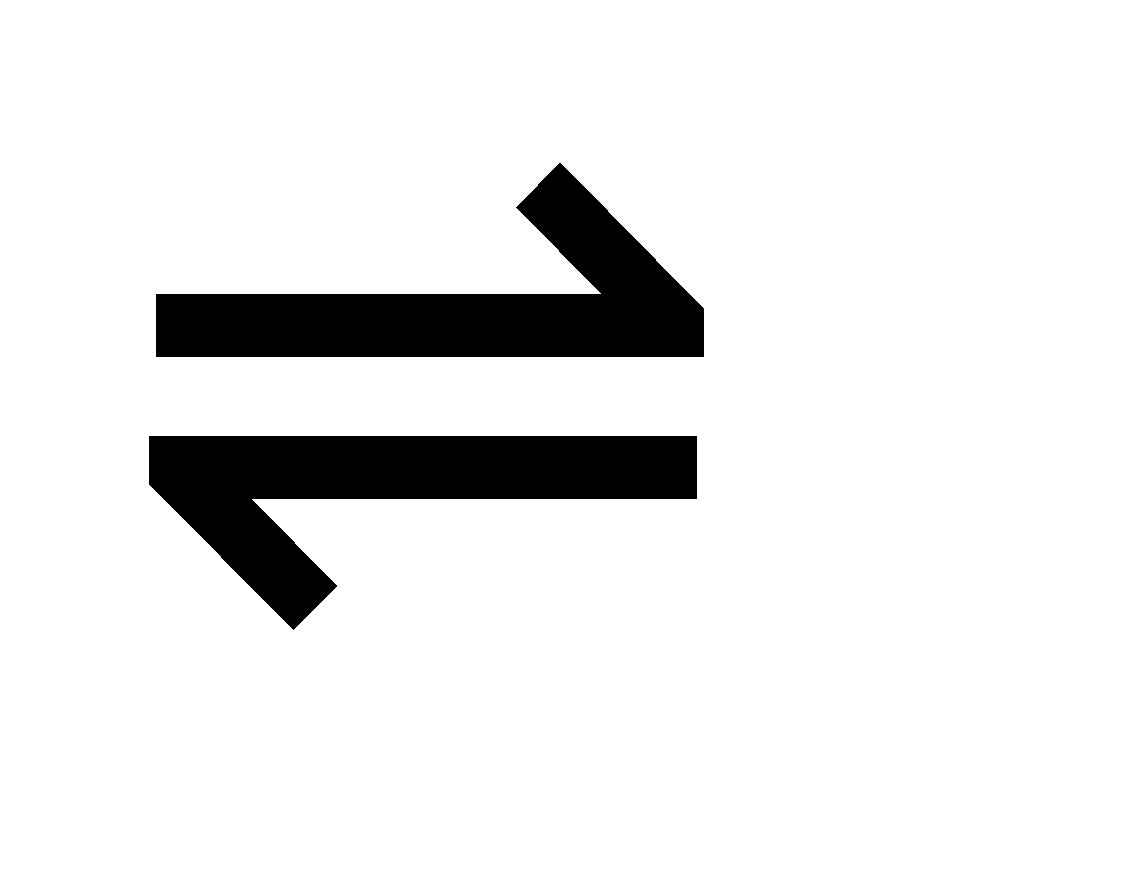 water (l)
water (l)  vapour (g) can exist at 0.0098°C and 4.58 mm.
vapour (g) can exist at 0.0098°C and 4.58 mm.
DEGREE OF DISSOCIATION FROM DENSITY MEASUREMENT
The density of one mole of gas is given by
D = 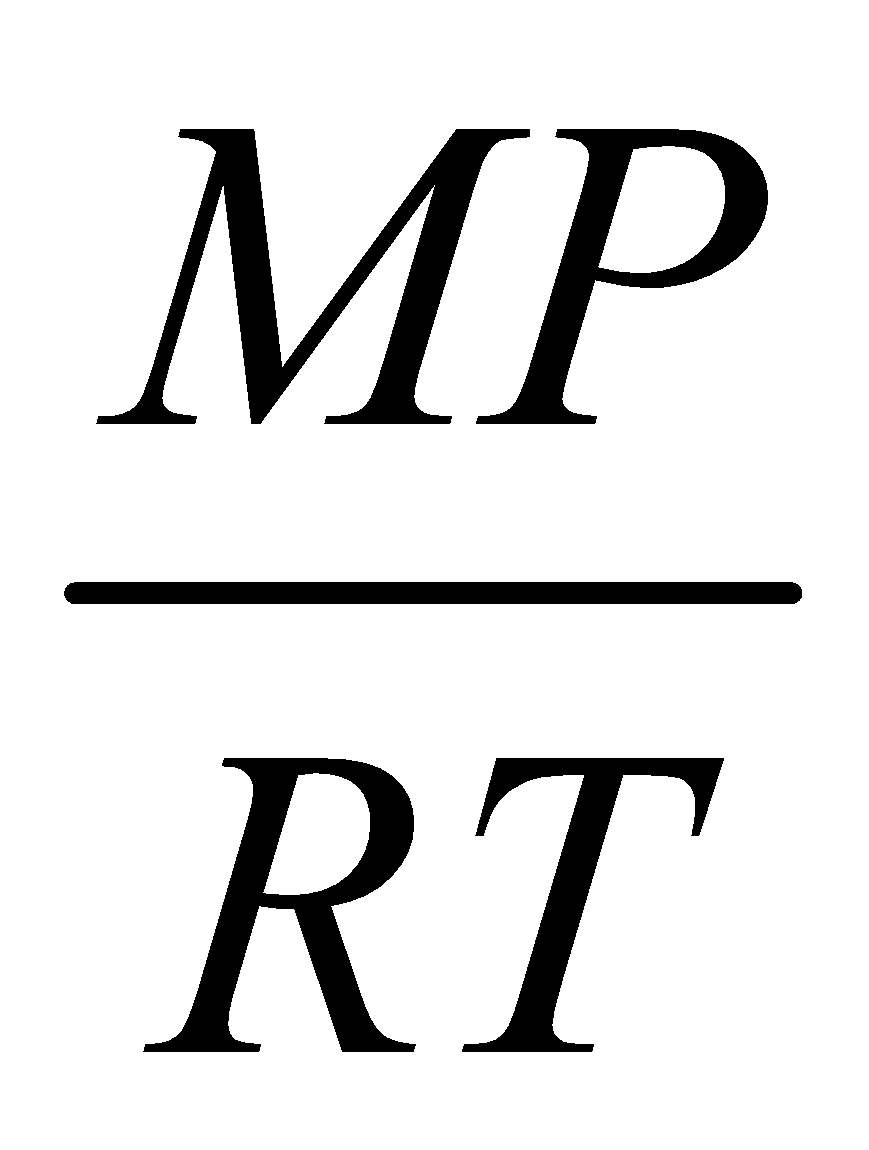 where M = Mol. wt of gas; P = Total pressure.
where M = Mol. wt of gas; P = Total pressure.
The volume of the gas increases on dissociation in proportion to increase in the total number of moles, but total weight remains constant. Hence density decreases in the same proportion. Consider dissociation of PCl5. Let x be degree of dissociation
PCl5  PCl3 + Cl2
PCl3 + Cl2
1 – x x x Total moles (1 + x)
where D is the theoretical vapour density and
D= 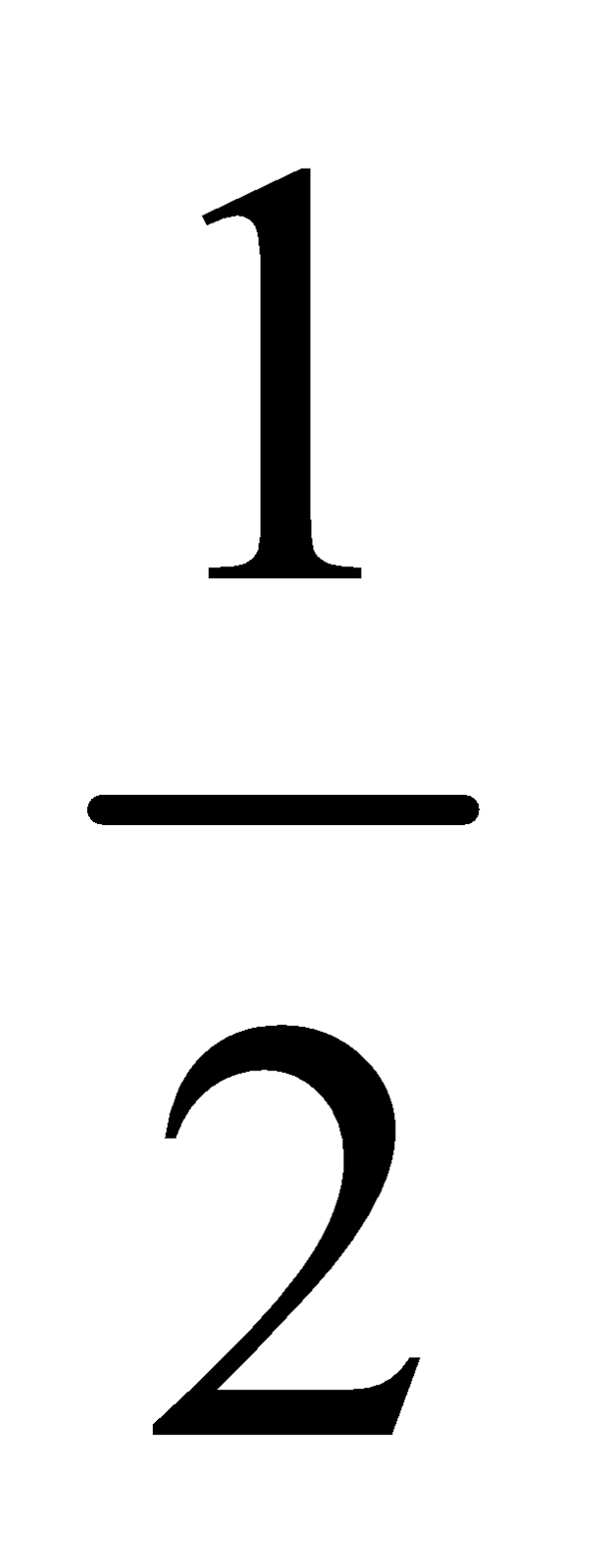 Molecular mass
Molecular mass
d is observed vapour density at temperature tºC.
If nx moles of products are formed, then total number of moles after dissociation
1 – x + nx = 1 + x (n – 1)
