Question
The amount of a drug, in milligrams (mg), in a patient’s body can be modelled by the function
A(t) = 500\(e^{-kt}\), where k is a positive constant and t is the time in hours after the initial dose is given.
(a) Write down the amount of the drug in the patient’s body when t = 0.
After three hours, the amount of the drug in the patient’s body has decreased to 280mg.
(b) Find the value of k .
The second dose is given T hours after the initial dose, when the amount of the drug in the patient’s body is 140mg.
(c) Find the value of T.
▶️Answer/Explanation
Answer:
(a) A(0) = 500 (mg)
(b) 280 = 500\(e^{-3k}\)
k = 0.193272…
k = 0.193(=-\(\frac{1}{3}In(\frac{380}{500}\)))
(c) 500\(e^{-0.193272…T}\) = 140
T = 6.58636…
T = 6.59 (h)
Question 6. [Maximum mark: 7]
All living plants contain an isotope of carbon called carbon-14. When a plant dies, the isotope
decays so that the amount of carbon-14 present in the remains of the plant decreases. The
time since the death of a plant can be determined by measuring the amount of carbon-14 still
present in the remains.
The amount, A, of carbon-14 present in a plant t years after its death can be modelled by \(A=A_{0}e^{-kt}\) where \(t\geq 0\) and \(A_{0}\) ,k are positive constants.
At the time of death, a plant is defined to have 100 units of carbon-14.
(a) Show that \(A_{0} = 100\). [1]
The time taken for half the original amount of carbon-14 to decay is known to be 5730 years.
(b) Show that \(k=\frac{\ln 2}{5730} \) [3]
(c) Find, correct to the nearest 10 years, the time taken after the plant’s death for 25% of the carbon-14 to decay.
Answer/Explanation
(a) \(100=A_{0}e^{0}\)
\(100=A_{0}\)
(b) correct substitution of values into exponential equation
\(500=100e^{-5730k}\) OR \(e^{-5730k}=\frac{1}{2}\)
EITHER
\(-5730K=\ln \frac{1}{2}\)
\(\ln \frac{1}{2}=-ln2\) OR \( -\ln \frac{1}{2}=\ln 2\)
OR
\(e^{5730k}=2\)
\(5730k=\ln 2\)
THEN
\(k=\frac{\ln 2}{5730}\)
(c) if 25% of the carbon-14 has decayed,75 remains i.e, 75 units remain \(75=100e^{-\frac{\ln 2}{5730}t}\)
EITHER
using an appropriate graph to attempt to solve for t
OR
manipulating logs to attempt to solve for t
\(\ln 0.75=-\frac{\ln 2}{5730}\)
y=2738.164…
THEN
t=2380 (years)(correct to the nearest 10 years)
Question
It has been suggested that in rowing competitions the time, \(T\) seconds taken to complete a 2000 m race can be modelled by an equation of the form \(T = a{N^b}\), where \(N\) is the number of rowers in the boat and \(a\) and \(b\) are constants for rowers of a similar standard.
To test this model the times for the finalists in all the 2000 m men’s races at a recent Olympic games were recorded and the mean calculated.
The results are shown in the following table for \(N = 1\) and \(N = 2\).
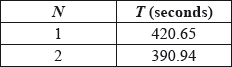
It is now given that the mean time in the final for boats with 8 rowers was 342.08 seconds.
Use these results to find estimates for the value of \(a\) and the value of \(b\). Give your answers to five significant figures.
Use this model to estimate the mean time for the finalists in an Olympic race for boats with 8 rowers. Give your answer correct to two decimal places.
Calculate the error in your estimate as a percentage of the actual value.
Comment on the likely validity of the model as \(N\) increases beyond 8.
Answer/Explanation
Markscheme
\(a = 420.65\) A1
\(390.94 = a \times {2^b}\) M1
\({2^b} = \frac{{390.94}}{{420.65}} = 0.929 \ldots \) A1
\(b = – 0.10567\) A1
[4 marks]
\(N = 8\,\,\,T = 337.67\) A1
Note: Accept 5sf answers between 337.44 and 337.67.
[1 mark]
\(N = 8\) Percentage error 1.29% A1
Note: Accept negative values of the above.
[1 mark]
likely not to be a good fit for larger values of \(N\) R1
likely to be quite a good fit for values close to 8 R1
[2 marks]
Examiners report
Parts (a) to (c) were generally well done, although far too much inaccuracy with basic calculations.
Parts (a) to (c) were generally well done, although far too much inaccuracy with basic calculations.
Parts (a) to (c) were generally well done, although far too much inaccuracy with basic calculations.
Parts (a) to (c) were generally well done, although far too much inaccuracy with basic calculations. Part (d) caused more difficulties as candidates frequently had insufficient analysis to gain the two marks.
