IB PHYSICS HL(Higher level) – 2024 – Practice Questions- All Topics
Topic 7.2 Nuclear reactions
Topic 7 Weightage : 3 %
All Questions for Topic 7.2 – The unified atomic mass unit , Mass defect and nuclear binding energy , Nuclear fission and nuclear fusion
Question
What is the relation between the value of the unified atomic mass unit in grams and the value of Avogadro’s constant in 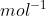 ?
?
A Their ratio is 1.
B Their product is 1.
C Their sum is 1.
D Their difference is 0.
▶️Answer/Explanation
Ans: B
unit which is widely used in describing mass in nuclear physics as well as in atomic physics is unified atomic mass unit denoted by the symbol \(u\). It is 1/12 of the mass of a neutral carbon atom in its lowest energy state which contains six protons, six neutrons and six electrons. We have
\(1u=1.66\times 10^{-27}kg= 931 MeVc^{-2}\)
Avogadro’s number is defined as the total number of atoms in \(12 g\) sample of \(carbon -12\)
so, \(12g \;_{}^{12}\textrm{C} \) contain \(N_A\) atoms
Therefore, 1 atom of carbon -12 weighs \(\frac{12}{N_A}g\)
As per the definition of atomic mass unit
\(1 amu = \frac{1}{12}\times \frac{12}{N_A}g\)
\(= \frac{1}{6.022 \times 10^{23}}g\)
Hence , \(1 amu = 1.6 \times 10^{-27}kg\)
Question
In the nuclear reaction X + Y → Z + W, involving nuclides X, Y, Z and W, energy is released. Which is correct about the masses (M) and the binding energies (BE) of the nuclides?
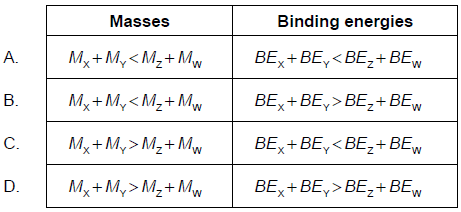
▶️Answer/Explanation
Markscheme
C
Greater the binding energy of a substance more stable it will be. Reactants will react to convert into products if the products formed will be more stable than the reactants. So binding energy of products is more than the reactants. This extra Stability is achieved by mass defect ( loss in mass) which is converted in Energy.
Question
Patterns in graphs help scientists make predictions. What can be deduced from a graph of neutron number versus proton number for all stable nuclides?
A. The short-range nature of the strong nuclear force
B. The increase of the binding energy per nucleon with proton number
C. The existence of quarks and leptons
D. The existence of alpha decay
▶️Answer/Explanation
Markscheme
A
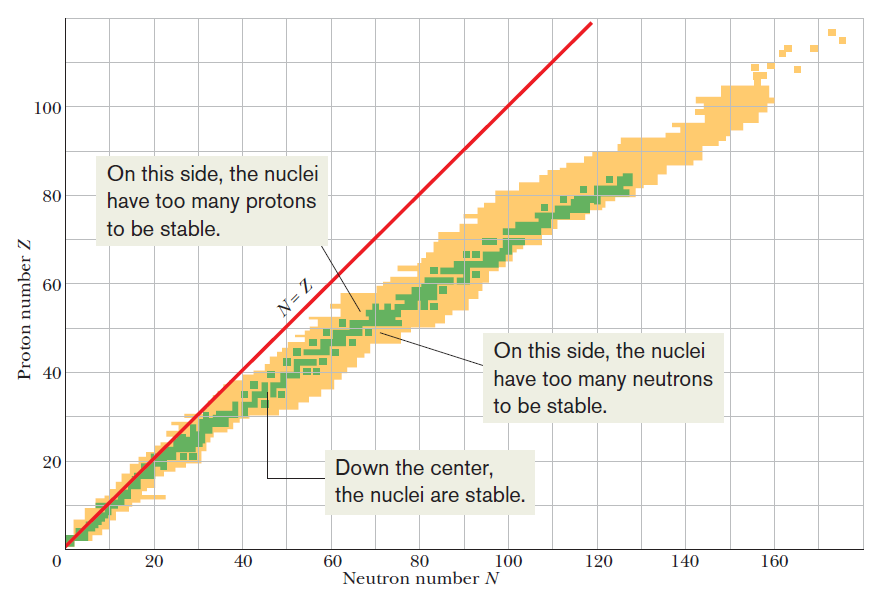
The nuclear strong force is about 100 times as strong as the electrostatic repulsions.
It operates over only short distances.
After a certain size, the strong force is not able to hold the nucleus together.
Adding extra neutrons increases the space between the protons.
This decreases their repulsions but, if there are too many neutrons, the nucleus is again out of balance and decays.
Question
A fission reaction for uranium is
\[{}_{92}^{235}{\rm{U}} + {\rm{n}} \to {}_{56}^{141}{\rm{Ba}} + {}_Z^A{\rm{Kr}} + 3{\rm{n}}\]
where n is the neutron. Which of the following gives the value of the nucleon number A and proton number Z for the krypton (Kr)?
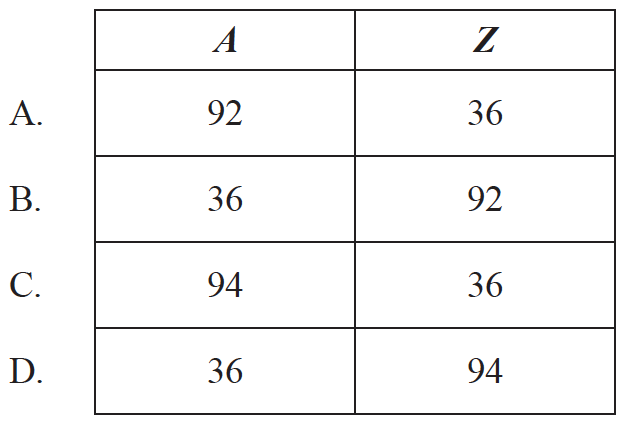
▶️Answer/Explanation
Markscheme
A
\({}_{92}^{235}\textrm{}U+_{0}^{1}\textrm{}n\rightarrow _{56}^{141}\textrm{}{Ba}+_{Z}^{A}\textrm{}{Kr}+3_{0}^{1}\textrm{}n\)
Balancing Number of proton
\(92+0=56+Z+0 \Rightarrow Z =36\)
Balancing Mass Number \(A\)
\(235+1=141+A+3 \times 1 \Rightarrow A =92\)
Question
The graph shows the relationship between binding energy per nucleon and nucleon number. In which region are nuclei most stable?
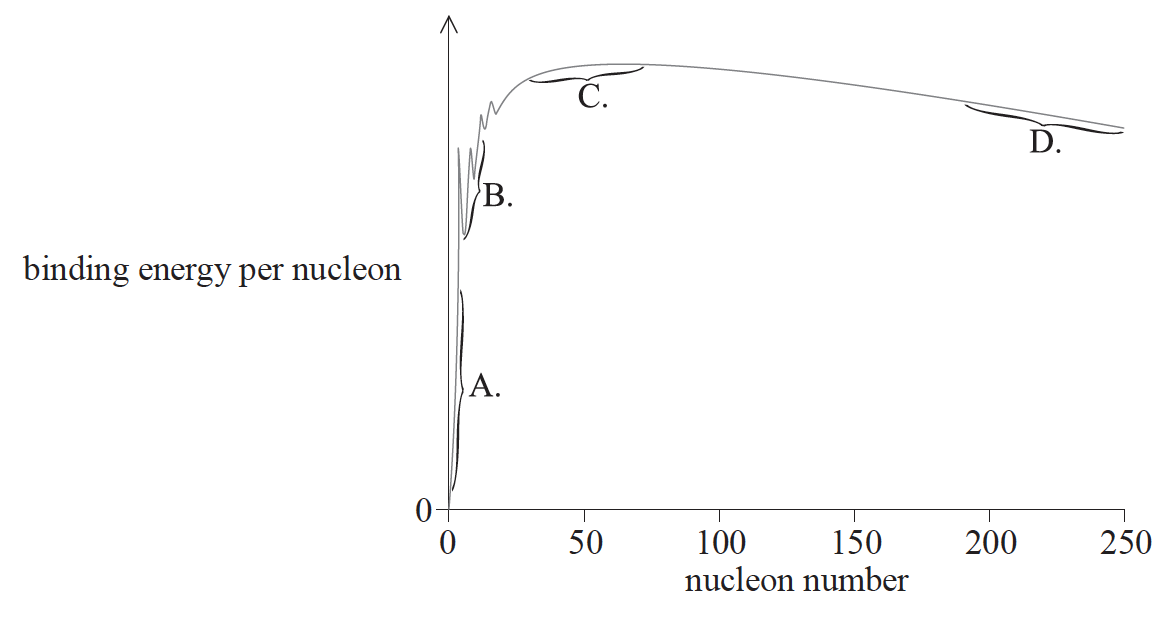
▶️Answer/Explanation
Markscheme
C
Question
The nuclear reaction represented by
\[{}_0^1{\rm{n + }}{}_{92}^{235}{\rm{U}} \to {}_{56}^{141}{\rm{Ba + }}{}_{36}^{92}{\rm{Kr + 3}}{}_0^1{\rm{n}}\]
is an example of
A. nuclear fusion.
B. nuclear fission.
C. artificial transmutation.
D. radioactive decay.
▶️Answer/Explanation
Markscheme
B
