IBDP Physics- E.1 Structure of the atom - IB Style Questions For SL Paper 1A -FA 2025
Question
▶️ Answer/Explanation
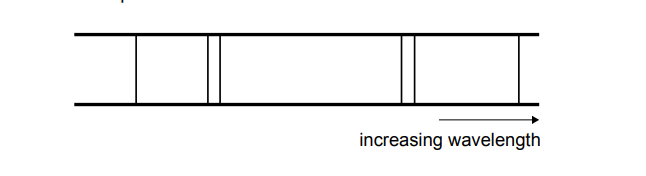
1. Analyze Spectrum:
The spectrum shows lines that get closer together (converge) as wavelength decreases (frequency/energy increases). This convergence is characteristic of electron transitions.
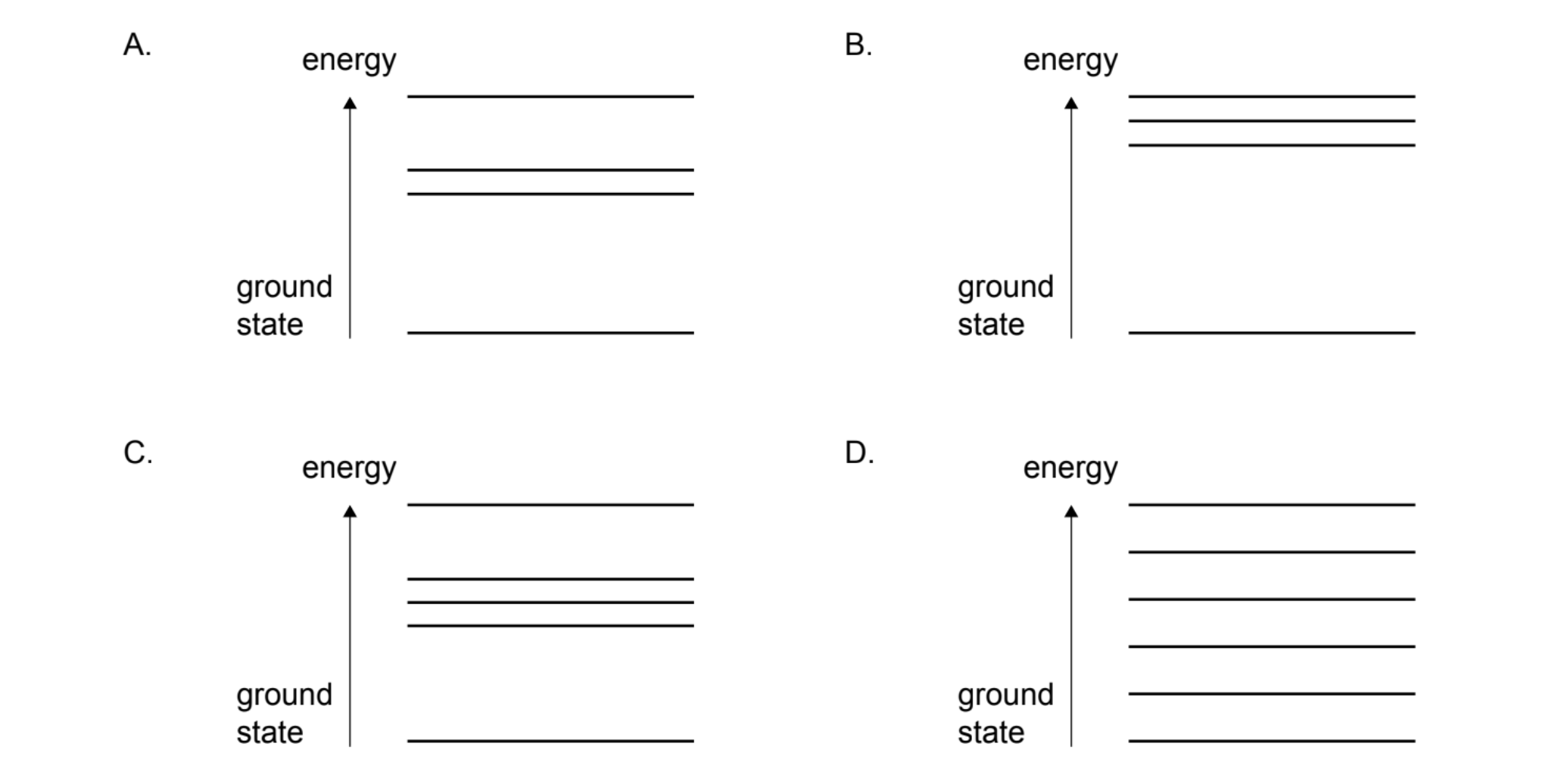
2. Analyze Energy Levels:
In an atom, energy levels converge at higher energies (further from the nucleus). The gaps between levels get smaller as \(n\) increases.
3. Relate to Transitions:
Emission lines correspond to transitions from higher levels to a lower level. Since the levels themselves converge at the top, the differences in energy between adjacent transitions also change in a specific pattern.
Diagram A shows levels converging at higher energy (away from the ground state). This is the standard model for atomic energy levels (e.g., Hydrogen).
✅ Answer: (A)
Question
II. The positive charge of the atom is concentrated in a small volume.
III. The electrons have discrete energy levels.
(B) I and III only
(C) II and III only
(D) I, II and III
▶️ Answer/Explanation
In the Rutherford–Geiger–Marsden scattering experiment, most \(\alpha\)-particles passed straight through the foil with little or no deflection.
This indicates that most of the atom is empty space (Claim I).
A small fraction of \(\alpha\)-particles were deflected through large angles (some even back-scattered).
This implies the positive charge (and most of the mass) is concentrated in a very small region, the nucleus (Claim II).
The experiment does not provide evidence that electrons have discrete energy levels.
Discrete energy levels are supported by line spectra and later atomic models (Claim III is not deduced here).
✅ Answer: (A)
Question
(B) the existence of the neutron.
(C) a dense positively charged nucleus.
(D) the stability of some nuclei.
▶️ Answer/Explanation
In the Geiger–Marsden (Rutherford scattering) experiment, most \(\alpha\)-particles passed straight through the foil, but a small fraction were deflected through large angles.
Large deflections are only possible if the atom’s positive charge (and most of its mass) is concentrated in a very small region, meaning the nucleus is dense and positively charged.
✅ Answer: (C)
