Question:
Complete the following table to determine the number of valence electrons and electron configuration for the following elements. The first one has been done for you.
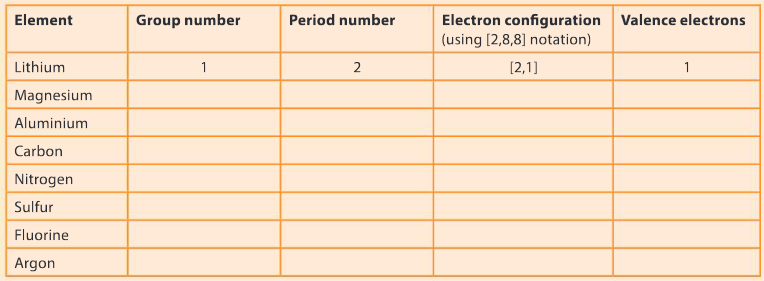
▶️Answer/Explanation
Ans: 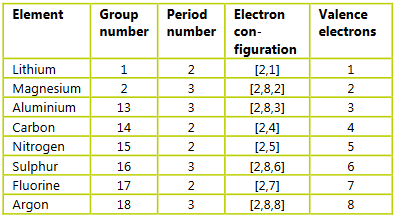
Question:
Determine the full electron configuration for the following atoms and ions:
a) sodium (Z = 11) b) aluminium (Z = 13)
c) chlorine (Z = 17) d) vanadium (Z = 23)
e) copper (Z = 29) f) bromine (Z = 35)
g) oxide ion, O2 ̄ (for h) magnesium ion, Mg2+
elemental oxygen, (for elemental
Z = 8) magnesium, Z = 12)
i) sulfide ion, S2 ̄ (for j) potassium ion, K+ (for
elemental sulfur, Z = 16) elemental potassium,
Z = 19)
▶️Answer/Explanation
Ans: a) 1s22s22p63s1
b) 1s22s22p63s23p1
c) 1s22s22p63s23p5
d) 1s22s22p63s23p64s23d3
e) 1s22s22p63s23p64s13d10
f) 1s22s22p63s23p64s23d104p5
g) 1s22s22p6
h) 1s22s22p6
i) 1s22s22p63s23p6
j) 1s22s22p63s23p6
Question:
How can we use the value of the ionization energy of an atom as an indicator of the level of reactivity of an element?
▶️Answer/Explanation
Ans: The lower the ionization energy of an element, the more able it is to share electrons, which indicates higher reactivity.
Question:
What factors determine the relative size of the ionization energy of an element?
▶️Answer/Explanation
Ans: The size of the effective nuclear charge (which depends on the group number) and the level of shielding provided by the electron shells (which depends on the period number).
Question:
A campfire generates radiant heat that will keep you warm on a cold winter’s night. Additional wooden logs will increase the amount of radiant heat produced. Using this analogy, what happens to the nucleus of an atom as the number of protons increases?
▶️Answer/Explanation
Ans: The charge on the nucleus increases.
Question:
After discussing your results with other students, explain why there is no reaction between chlorine water and potassium chloride.
▶️Answer/Explanation
Ans: Molecular chlorine cannot displace the chloride ion from solution as they are the same element.
Question:
Based on your initial observations of the color of chlorine, bromine and iodine water, draw conclusions about the color changes you observed in each of the individual reactions.
▶️Answer/Explanation
Ans: In the reaction between chlorine and potassium bromide, the colour change observed is from yellow/green to a red/brown, indicating a reaction has occurred and bromine has been formed.
In the reaction between chlorine and potassium iodide, the colour change observed is from yellow/green to a dark brown, indicating that a reaction has occurred and iodine has been formed.
In the reaction between bromine and potassium iodide, the colour change observed is from red/brown to dark brown, indicating that a reaction has occurred and iodine has been formed.
All the remaining combinations do not result in a reaction.
Question:
Construct balanced equations for the reactions which occurred during the experiment.
▶️Answer/Explanation
Ans: Cl2(aq) + 2KBr(aq) → Br2(aq) + 2KCl(aq)
Cl2(aq) + 2KI(aq) → I2(aq) + 2KCl(aq)
Br2(aq) + 2KI(aq) → I2(aq) + 2KBr(aq)
Question:
Analyze the pattern in the reactivity of the individual halides and propose an order of reactivity for the halogens based on experimental evidence.
▶️Answer/Explanation
Ans: Cl > Br > I
Question:
Spectator ions are unchanged on both the reactant and product side of the chemical equation. Remove the spectator ions and write ionic equations for each of the reactions.
▶️Answer/Explanation
Ans: Cl2(aq) + 2Br–(aq) → Br2(aq) + 2Cl–(aq)
Cl2(aq) + 2I–(aq) → I2(aq) + 2Cl–(aq)
Br2(aq) + 2I–(aq) → I2(aq) + 2Br–(aq)
Question:
Consider the reactions of the following Group 3 oxides with water: sodium oxide, aluminium oxide, and sulfur dioxide. Describe whether each is acting as an acid or base. What does this suggest about the change in acid–base properties across a period?
▶️Answer/Explanation
Ans: Sodium oxide is acting as a basic oxide; aluminium oxide is amphoteric and can act as both a basic and acidic oxide; sulfur dioxide is acting as an acidic oxide; the general trend across period 3 of the periodic table is a change from basic to acidic oxides.
Question:
Construct a table and record your observations. Include the following:
a) the name and symbol of the reactants of each reaction
b) a description of the reaction including the color of any flame produced when the reactant was heated
c) the initial and final color
d) pH of the final reaction mixture.
▶️Answer/Explanation
Ans: 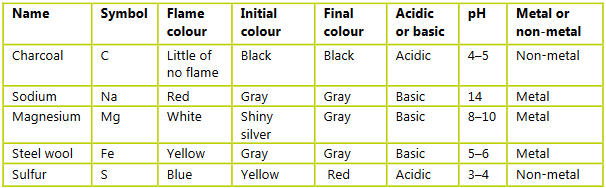
Question:
Using a universal indicator color strip, estimate the pH of each solution and record it in your data table.
▶️Answer/Explanation
Ans: 
Question:
Separate the reactants into two groups: metals and non-metals. Can you identify a trend in the pH values for each of these groups?
▶️Answer/Explanation
Ans: 
Question:
Write a general statement to describe the acid–base nature of metallic oxides and non-metallic oxides.
▶️Answer/Explanation
Ans: The acidity of oxides increases across a period, with non–metallic oxides more acidic than metallic oxides.
Summative assessment
Patterns in physical and chemical properties
You are going to analyze and evaluate the trends in data for the first ionization energy (kJ mol−1) of the first 20 elements of the periodic table.
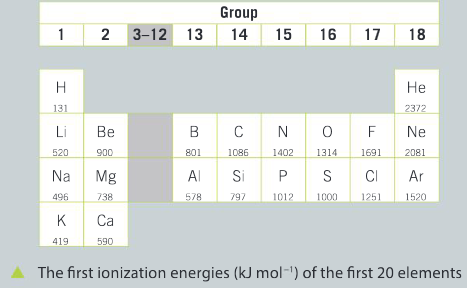
Question:
Identify elements with the lowest first ionization energy. What do you observe about the group they come from in the periodic table?
▶️Answer/Explanation
Ans: Hydrogen, lithium, sodium and potassium have the lowest first ionization energies; they are all members of group 1, the alkali metals.
Question:
Explain why the elements identified above tend to lose an electron.
▶️Answer/Explanation
Ans: Alkali metal elements have one valence electron in the outermost electron shell; the shielding effect of the core electrons means that the valence electron is weakly attracted to the positively charged nucleus; Group 1 metals like to lose the one valence electron to obtain a stable noble gas configuration which is equivalent to a full outer electron shell.
Question:
Identify the elements with the highest first ionization energy.
a) Are they all members of the same group?
▶️Answer/Explanation
Ans: Helium, neon, argon and fluorine have the highest first ionization energies. Helium, neon and argon are all in group 18, with eight valence electrons, and fluorine is in group 17, with seven valence electrons.
b) Explain why it is so difficult to remove an electron from these elements.
▶️Answer/Explanation
Ans: As you move across a period the effective nuclear charge increases as the number of protons in the nucleus increases while electrons are filling orbitals of the same energy level; the valence electrons of the noble gases are strongly attracted to the nucleus and these elements are very stable and tend not to lose or gain electrons.
Question:
a) State the trend in first ionization energies as you move down a group in the periodic table.
▶️Answer/Explanation
Ans: As you move down a group, the first ionization energy of an element decreases.
b) Suggest reasons why this trend in ionization energies exists.
▶️Answer/Explanation
Ans: The atomic radii of an atom increases as you move down a group; the level of shielding of the valence electrons by core electrons increases as you move down a group; the combination of these two factors means that the attraction between the positive nucleus and the valence electrons decreases down the group, and therefore the ionization energy decreases.
The following chart shows the melting and boiling points of the first 20 elements of the periodic table.
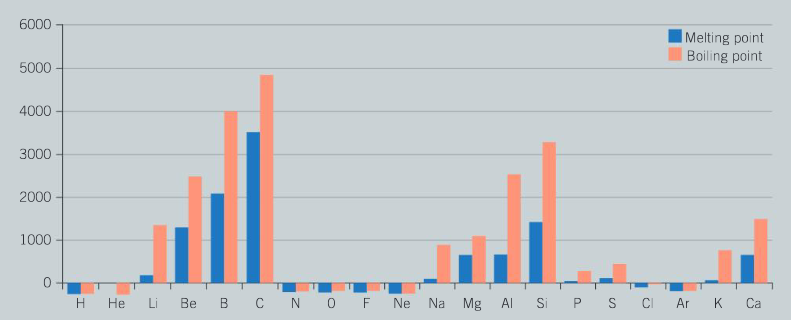
Question:
Examine the graph and describe the pattern in melting points as you move from left to right across period 3 (from sodium to argon).
▶️Answer/Explanation
Ans: There is an increase in melting point of the first three metals (Na, Mg, Al) as the strength of metallic bonding increases; the sudden increase in melting point for silicon is due to the fact that this is a giant covalent structure with very high melting points and boiling points due to the strong covalent bonding; the large decrease in melting points of the final elements of period 3 is due to the fact that these elements (P, S, Cl, Ar) are simple molecular substances which are held together by weak intermolecular forces between the molecules.
Question:
Chemists often refer to metals and non-metals when describing elements in the periodic table. Explain how you can identify metals and non-metals by examining patterns in the melting points of the individual elements.
▶️Answer/Explanation
Ans: Metals tend to have high melting points and boiling points as a result of strong metallic bonding; and are solids at room temperature (except for mercury); non–metals are mainly gases and liquids at room temperature as a change in state for a molecular compound involves the breaking of weak intermolecular forces between the molecules.
Question:
Which group of the periodic table has the maximum values for their melting points? Identify the type of bonding present in these elements and explain why the melting points are so high.
▶️Answer/Explanation
Ans: Group 14 has the maximum values for melting points; the elements carbon (diamond and graphite) and silicon exist as giant covalent lattice structures; the very high melting points are a consequence of the large number of strong covalent bonds within the structure.
Patterns in the thermal conductivity of a metal
Thermal conductivity is the rate of movement of heat through a material. By observing patterns in the way materials conduct heat, chemists have been able to classify specific groups of elements and compounds as high, medium and low thermal conductivity materials. Materials with low thermal conductivity are thermal insulators and materials with high thermal conductivities are used in applications that exploit this. In general, metallic materials have high electrical and thermal conductivity because of their metallic bonding.
The following is a method proposed by a student to test the thermal conductivity of a series of metals.
| Materials ● 30 cm rods of copper, steel (iron), aluminium, brass and zinc ● Bunsen burner ● Retort stand and cork-lined clamp ● Stopwatch ● Wax candle and thumbtack Method ● Secure a clamp to the end of one of the metal rods. ● Fix the clamp in place on the retort stand. ● Light a candle and drop some liquid wax onto the rod near to the clamp. Press the base of the thumbtack into the liquid wax and hold it until the liquid wax cools and the thumbtack is secured. ● Position the Bunsen burner at the opposite end to the thumbtack. ● Measure and record the distance between the thumbtack and the Bunsen burner. ● Light the Bunsen burner, adjust the flame to maximum and start the stopwatch. ● When the wax melts and the thumbtack falls from the metal rod, stop the stopwatch. ● Record your qualitative and quantitative observations. ● Repeat the procedure for each of the remaining metals. |
Question:
Consider the method outlined above and suggest a possible hypothesis that the student was exploring.
▶️Answer/Explanation
Ans: Answers to this question will vary. Any plausible hypothesis that can be supported by the accepted scientific context is acceptable.
Question:
Evaluate the method and identify the independent, dependent and control variables.
▶️Answer/Explanation
Ans: Independent variable: type of metal; dependent variable: thermal conductivity/time taken for the thumbtack to drop; control variables: length of metal rod, position of the Bunsen burner flame on the metal rod, cross– sectional area of the rod.
Question:
Discuss whether sufficient relevant data will be generated by this method.
▶️Answer/Explanation
Ans: The methodology suggests that only one trial is undertaken for each of the four different metals; insufficient experimental data will be collected.
Question:
Explain how the method could be improved or extended.
▶️Answer/Explanation
Ans: Additional trials be conducted so that raw data can be averaged and anomalies identified; a more precise method for determining the rate of thermal conductivity such as temperature sensors attached to the furthest end of the metal rod; additional types of metals can be used in the experiment.
Analyzing the periodic properties of the halogens
The German scientist Döbereiner developed the law of triads during the 1820s. His experiments and observations suggested that the chemical and physical properties of bromine were intermediate to those of chlorine and iodine; which we now know are, respectively, positioned above and below bromine in the periodic table. A lack of experimental evidence about different triads within the periodic table meant that his findings were regarded by the scientific community of the time as interesting rather than groundbreaking.
A student performs a series of experiments to investigate the periodic properties of group 17, the halogens. The results and observations from the experiment are presented below.
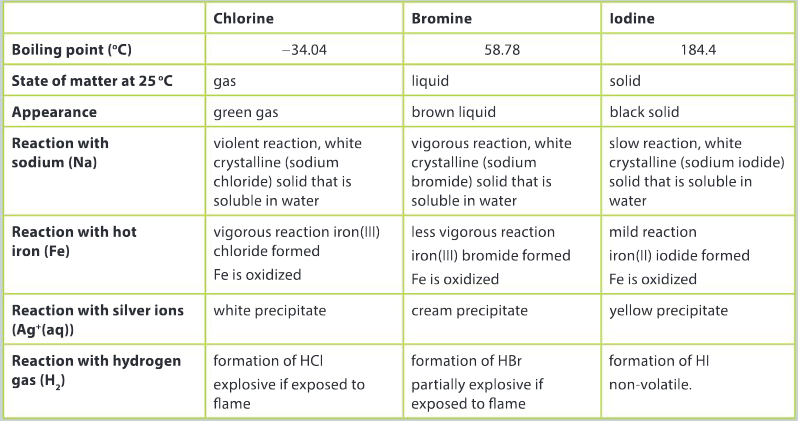
Question:
Interpret the data and use your knowledge of patterns in physical and chemical properties to list arguments for placing these elements in the same group in the periodic table.
▶️Answer/Explanation
Ans: There is a trend in the state of matter down the group; the colour of the halogens darkens progressively down the group; boiling points are low, typical of non–metals; all three elements in the reaction with sodium metal produce a white crystalline solid that is soluble in water; all three elements react with iron to form an iron halide compound and the level of reactivity progressively decreases down the group; the colour of silver halide precipitates darkens down the group.
Question:
Explain how the student could extend or improve this investigation into the halogens.
▶️Answer/Explanation
Ans: Additional experiments to support the observation of gradual trends in data down the group; examination of trends in other physical properties such as melting point; perform displacement reactions to determine the trend in the reactivity of the halogens.
Patterns in nature
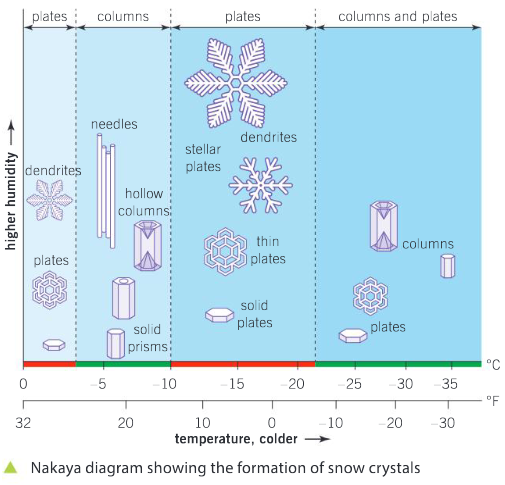
With reference to the Nakaya diagram, consider the following questions.
Question:
Identify the conditions necessary for large dendrite plate-shaped crystals to form. You should state both the temperature range and hollow columns.
▶️Answer/Explanation
Ans: For large dendrite plate–shaped crystals to form, a temperature range of –10°C to –22°C; and high humidity are required.
Question:
Identify and state the temperature at which needle-shaped crystals form.
▶️Answer/Explanation
Ans: –3°C to –10°C or 28°F to 13°F
Question:
Which requires more humidity to form: solid plates or hollow columns?
▶️Answer/Explanation
Ans: Hollow columns; requires higher humidity and a lower temperature.
Question:
Suggest reasons why the work of Nakaya was so important to the field of crystal research.
▶️Answer/Explanation
Ans: The work of Japanese physicist Ukichiro Nakaya was very important as he described and classified snow crystals; he provided fellow scientists with a methodology; to discover describe and classify new examples.
Question:
Create a detailed and well-labelled diagram, flowchart or other visual means to explain the formation of a snow crystal.
▶️Answer/Explanation
Ans: Visual representations should include the following features: temperature conditions at which the snowflake forms; level of relative humidity; height of the clouds; air currents; path the crystals take through the clouds; diagram presented and labelled clearly.
