Question:
List as many examples as you can of water in each of its three states.
▶️Answer/Explanation
Ans: Solid: Ice cubes, snow, icebergs
Liquid: Rainwater, tap water, oceans
Gas: Steam, water vapor in humid rainforests
Question:
What type of bonding is found within a water molecule, between the oxygen and hydrogen atoms?
▶️Answer/Explanation
Ans: Covalent bonding.
Question:
Name and explain the intermolecular force that exists between molecules of water.
▶️Answer/Explanation
Ans: Hydrogen bonding exists between molecules of water. It is the attraction between the electronegative oxygen atoms of one water molecule and the electropositive hydrogen atoms of another.
Question:
Why is the density of ice lower than that of liquid water? Give an example of where this can be observed on our planet.
▶️Answer/Explanation
Ans: Water molecules in liquid water are more closely packed than water molecules in solid ice, due to greater hydrogen bonding between the molecules. This is observed when ice cubes float in your drink, or when icebergs float on the surface of the ocean; for a solid object to float in a liquid, it must have a lower density than the liquid.
Question:
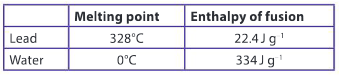
a) How much energy does it take to melt 1.5 kg of water that is at its melting point?
▶️Answer/Explanation
Ans: Heat of fusion = Q / m
Q = Heat of fusion × mass = 334 J g–1 × 1500 g
= 501000 J
= 501 kJ
b) How much energy does it take to melt 1.5 kg of lead that is at its melting point?
▶️Answer/Explanation
Ans: Q = Heat of fusion × mass = 22.4 J g–1 × 1500 g
= 33600 J
= 33.6 kJ
Question:
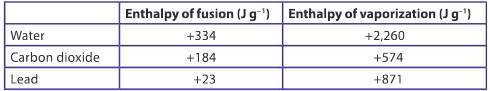
Compare these values of enthalpies of fusion and vaporization. Explain why the enthalpy of vaporization for a substance is greater than its enthalpy of fusion.
▶️Answer/Explanation
Ans: The enthalpy of fusion is the amount of energy required for the phase change from solid to liquid. Take water as an example. This energy is required to break the forces between molecules of water, allowing them to flow freely in the liquid state. The intermolecular forces still exist but they have been weakened. The enthalpy of vaporization is the energy required for the phase change from a liquid to a gas. This requires the intermolecular forces to be completely broken and for the molecules to have sufficient kinetic energy to overcome these forces of attraction.
Question:
Explain why the temperature of a boiling liquid does not continue to increase above its boiling point.
▶️Answer/Explanation
Ans: When the boiling point of a liquid is reached, some water molecules have sufficient kinetic energy to break the cohesive forces holding them to each other, in this case hydrogen bonding. When these energetic molecules leave the liquid water sample, they take with them a large amount of energy.
Question:
Steam burns are regarded as being far more harmful than a burn from boiling water. What change of state is occurring when steam reaches your skin? Why are the burns severe? Explain this in terms of energy transfer.
▶️Answer/Explanation
Ans: When steam comes into contact with your skin, the gaseous water will condense into liquid water. The high–energy water vapor releases a large amount of heat energy into your skin, resulting in a burn.
Question:
Explain why your skin feels cold when you leave the water after swimming on a hot summer day.
▶️Answer/Explanation
Ans: On a hot summer’s day, the water on your skin will begin to evaporate. Some of the energy required for the heat of vaporization will come from the Sun, while some will come from your skin. As the water molecules evaporate, they take energy with them, hence the reason why your skin feels cold.
Question:
Why does a boiling liquid immediately stop boiling the moment you remove the source of energy?
▶️Answer/Explanation
Ans: When you remove the heat source from boiling water, the water molecules that have sufficient energy continue breaking the intermolecular forces attracting them to each other for a short
period. They take with them large amounts of energy, which drops the temperature of the remaining liquid water molecules below the boiling point and hence the water stops boiling.
Question:
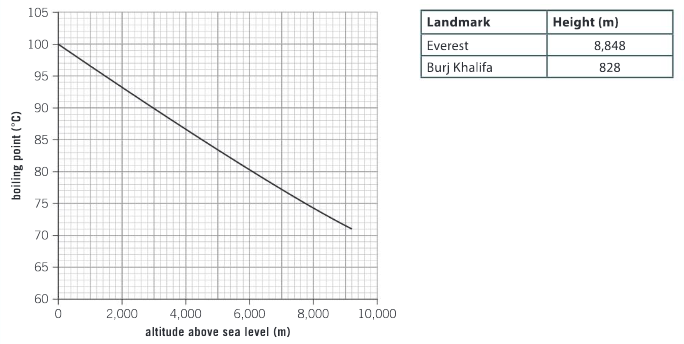
Study the graph and the table above. Use the information to calculate the percentage increase of the boiling point from the summit of Everest to the top of the Burj Khalifa.
▶️Answer/Explanation
Ans: Change in boiling point from Everest to Burj Khalifa is 97 – 72 = 25°C
% increase is 25 / 72 × 100% = 34.7%
Question:
Will it take less time to cook pasta at the top of the Burj Khalifa or the summit of Everest? Explain your answer. What do you expect to happen to the boiling point below sea level?
▶️Answer/Explanation
Ans: It will take less time to cook pasta at the top of Burj Khalifa as the temperature of boiling water on this mountain is 97°C. While it may take longer for the water to start boiling at the lower altitude, the boiling water is at a higher temperature and will cook the pasta faster. Water will boil at a temperature greater than 100°C below sea level.
Question:
At –78.5°C solid carbon dioxide (dry ice) sublimates. However, the white vapor you see is not carbon dioxide—it is a mist of water droplets condensed from the air. Explain the changes in state that occur.
▶️Answer/Explanation
Ans: When carbon dioxide sublimates, the gaseous carbon dioxide is at a very low temperature. When water vapor comes into contact with the cold carbon dioxide vapors, it causes the water vapor
to condense from the air. This change in state is from a gas to a liquid.
Question:
Write the word equation for the reaction between iron(III) nitrate and sodium carbonate.
▶️Answer/Explanation
Ans: iron(III) nitrate + sodium carbonate
↓
iron(III) carbonate + sodium nitrate
Question:
Write a balanced chemical equation for the reaction.
▶️Answer/Explanation
Ans: 2Fe(NO3)3(aq) + 3Na2CO3(aq)
↓
Fe2(CO3)3(s) + 6NaNO3(aq)
Question:
Using your knowledge of chemistry, examine the products of this reaction and predict the composition of the insoluble salt. Explain your reasoning.
▶️Answer/Explanation
Ans: From our work on salts, we know that all nitrate salts are soluble in water. Therefore, the insoluble precipitate that is formed during this reaction will be iron(III) carbonate.
Question:
Which of the following mixtures could be separated using filtration?
a) Sugar dissolved in water
▶️Answer/Explanation
Ans: As sugar dissolves in water, there is no insoluble matter to filter so this technique will not separate this mixture.
b) Sand and water
▶️Answer/Explanation
Ans: As sand is insoluble in water, filtration is an appropriate technique. The residue will be sand and the filtrate will be water.
Question:
Explain how you could separate out the sand from a mixture of salt and sand.
▶️Answer/Explanation
Ans: Step 1: Add water to the sand/salt mixture. The salt will dissolve in the water.
Step 2: Filter the mixture to separate the sand from the salt water.
Step 3: Collect the salt water filtrate and transfer to an evaporating dish. Warm the mixture and evaporate the water. The salt can now be recovered.
Question:
What types of liquids are typically separated using this technique?
▶️Answer/Explanation
Ans: Liquids that do not mix. For example polar liquids and non–polar liquids.
Question:
Explain why you need to remove the stopper prior to opening the stopcock.
▶️Answer/Explanation
Ans: If the stopcock remains in place while the liquid is draining from the separatory funnel, a vacuum will form and stop the liquids from leaving the vessel.
Question:
From your observations, can you infer the composition of the upper layer of the mixture? Explain your reasoning.
▶️Answer/Explanation
Ans: The upper layer is the less dense liquid. In this case it is the cooking oil. Your observations will tell you that the yellow colored liquid is the cooking oil.
Question:
Describe what happened to the boundary layer, as the volume of liquid remaining in the funnel decreased.
▶️Answer/Explanation
Ans: As the volume of liquid remaining in the funnel decreases, the cross–sectional area of the boundary phase decreases. This improves your ability to determine the boundary layer and separate the two different liquids more precisely.
Question:
What do you observe when you compare the colors of each of the successive fractions? Is there a pattern?
▶️Answer/Explanation
Ans: Each successive fraction is darker in colour.
Question:
Compare the flammability of each of the successive fractions. Which fraction is more flammable?
▶️Answer/Explanation
Ans: As the colour of the fraction darkens, the level of flammability decreases. The first fraction, the more volatile liquid, is more flammable.
Question:
What is the relationship between the boiling point and the flammability of the fraction? Explain your reasoning.
▶️Answer/Explanation
Ans: The liquids with the lower boiling point are more flammable. A liquid with a low boiling point is called a volatile liquid. The intermolecular forces are weak and the liquid will undergo a phase
change at a lower temperature. The vapors formed are flammable. Petrol is a good example of a flammable liquid. It has a low boiling point and is very flammable.
Summative assessment
Separation of mixtures
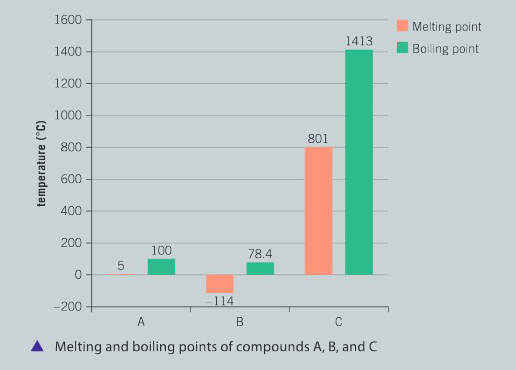
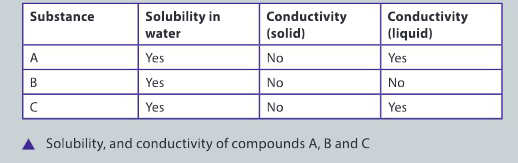
Physical mixtures of elements and compounds can be separated by a variety of techniques including filtration, fractional distillation, evaporation, chromatography and using a separatory funnel. The substances in a physical mixture are not chemically bonded to each other. Use the following information about three compounds (A, B, and C) to answer the questions.
Question:
Outline the following concepts.
a) Homogeneous mixture
▶️Answer/Explanation
Ans: A homogeneous mixture has both uniform composition and uniform properties throughout the mixture.
b) Heterogeneous mixture
▶️Answer/Explanation
Ans: A heterogeneous mixture has a non–uniform composition throughout the mixture and therefore its properties also vary.
Question:
State the most appropriate method for the physical separation of a mixture of compounds A, B and C. Justify your choice of technique.
▶️Answer/Explanation
Ans: Distillation is the most appropriate separation technique; Compound B has the lowest boiling point (78.4°C) and will be the first fraction to be collected; Compound A has second highest boiling point and will be the second fraction to be collected; Compound C has the highest boiling point; as Compound A is slowly removed, Com– pound C will remain behind in the flask and will crystallize; the rate of heating of the flask should be slow and controlled so that the separation is accurate and the purity of Compound A and B is high.
Question:
Determine the state of matter for each of the compounds A, B and C if the mixture is at a temperature of 80°C. Explain your reasoning.
▶️Answer/Explanation
Ans: Substance A has a melting point of 0°C and a boiling point of 100°C, therefore, will be a liquid at 80°C; substance B has a boiling point of 78.4°C, therefore will be a gas at 80°C; substance C has a melting point of 801°C, therefore, will be a solid at 80°C. [In the book, compound A should have a melting of 0°C not 5°C – Ed]
Question:
Predict the likely composition of substance C. Support your prediction with evidence.
▶️Answer/Explanation
Ans: Substance C is most likely to be an ionic compound such as sodium chloride; soluble in water, it breaks into its ions and is able to conduct an electric current, as a solid, there are no free ions and the electrons are localized, therefore it is a non–conductor; it has a high melting point and boiling point.
Question:
Identify the type of mixture containing compounds A, B and C. Explain your reasoning.
▶️Answer/Explanation
Ans: Based on qualitative observations and recorded data, the mixture is homogeneous; all three compounds are soluble in water; compound A has a low melting point and relatively low boiling point, is a non–conductive as a solid and can conduct an electric current as a liquid – this compound is the molecular compound, water; compound B has a low melting point and is volatile in that it boils at 78.4°C, it mixes with water and is a non–conductor as a liquid – this compound is the alcohol, ethanol; compound C has high melting and boiling points, soluble in water and a conductor as a liquid – this is the ionic compound, sodium chloride; at the molecular level, water and ethanol tend to cluster together resulting in a pseudo–homogeneous mixture.
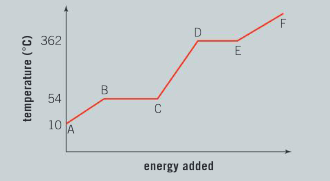
The graph illustrates the changes in state for myristic acid, a saturated fatty acid, as it is heated. Saturated acids have been linked to high levels of cholesterol in humans and an increased chance of coronary heart disease.
Question:
From your analysis of the graph:
a) Explain what is happening between B and C.
▶️Answer/Explanation
Ans: The region between b and c represents a solid/ liquid mixture; There is no increase in temperature in this region, as energy being added to the system is used to melt the solid myristic acid; when the change of state is complete and no more solid is present, the temperature will begin to rise again.
b) State the name used to describe this process.
▶️Answer/Explanation
Ans: Melting (or fusion).
c) Describe what is happening to the particles in this pure substance between C and D.
▶️Answer/Explanation
Ans: Heat energy is being added into the system from the surroundings resulting in an increase in temperature; the kinetic energy of the particles is increasing.
d) What is the boiling point of this liquid?
▶️Answer/Explanation
Ans: The boiling point of this liquid is 362°C.
e) The state of matter for this substance between E and F is a gas. Explain what will happen to the particles and the state of matter if energy is removed from the system.
▶️Answer/Explanation
Ans: If energy is removed from the system, the average kinetic energy of the particles decreases; particles move closer together and forces of attraction between the particles increases; there is a change of state from a gas to a liquid.
Identify an unknown salt
As a result of high humidity in the chemical storeroom, the labels on five separate reagent bottles were lost. You will need to design a series of analytical tests to identify their composition.

A selection of analytical tests for cations and anions is summarized below.
Question:
All of the salts are soluble in water. Design a series of tests to identify the composition of salts A–E. Your method should include a description of apparatus used and descriptions of the experimental techniques.
▶️Answer/Explanation
Ans: There are 5 different tests for which a method should be written. The tests are for cations, halide ions of chlorine, bromine and iodine, sulfate ion and the carbonate ion. Each method should describe the type and size of apparatus being used and the technique used to transfer solutions safely and precisely. [1 mark for each test]
Question:
Design a results’ table to accompany your experimental design
▶️Answer/Explanation
Ans: Data table should have the following features:
- Summary/identification of the tests performed for cations and for anions
- Qualitative observations for each test, including initial and final colors
- Assessment of whether the test is positive or negative
- Identification of the cation present
- Identification of the anion present
Marks awarded on a scale from 0 marks for a completely inadequate data table design to 10 marks for an exemplary design.
Analysis and evaluation
A chemist’s observations and results from these tests are shown below.
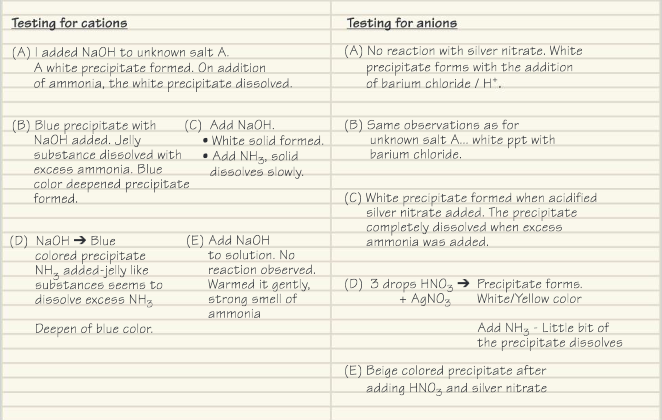
Question:
Present the data in an appropriate manner so that it can be used in a report.
▶️Answer/Explanation
Ans: Data table should have separate columns to present the name of the salt (A, B …); test performed; and the qualitative observations.
Question:
Identify the composition of the unknown salts A, B, C, D and E.
▶️Answer/Explanation
Ans: A Zinc(II) sulfate;
B Copper(II) sulfate;
C Zinc(II) chloride;
D Copper(II) bromide;
E Ammonium iodide;
The impact of palm oil
This article provides an overview of the benefits and problems of palm oil production.
Question:
Summarize how our increased scientific understanding of the impact of human development on the environment enables us to identify the issues that exist with palm oil production.
▶️Answer/Explanation
Ans: As previous biodiverse environments have been replaced by monocultures; a range of environmental problems have arisen: deforestation, soil erosion, smoke haze (from land clearing) and a reduction in animal and plant species.; the delicate balance of a vast range of ecosystems has been upset.
The features, and physical and chemical properties of different types of fats and oils are better understood as a consequence of the application of scientific research. Our ability to identify and analyze the chemical structure of substances we consume helps scientists to identify the connections between form and function.
Question:
Examine the iodine number of different oils used in food manufacturing and household cooking and determine which is considered the most healthy to consume. Explain the reasons for your answer.
▶️Answer/Explanation
Ans: From the information discussed in the article, soya bean oil is the healthiest oil to consume; it has a high iodine number (125–145) which means there is less saturated fat in this oil; Saturated fat can lead to increased cholesterol in the bloodstream and increased risk of heart disease and therefore is considered unhealthy.