Question (21 marks)
Developments in the understanding of atomic physics have led to many useful applications in industry, medicine and technology.
Through understanding patterns in the properties of alpha ( $\alpha)$, beta $(\beta)$ and gamma $(\gamma)$ radiation, scientists have developed ways in which the specific properties may be used.
One useful property is the difference in the penetration of alpha, beta and gamma radiation. The diagram compares the penetration of the three types of radiation.
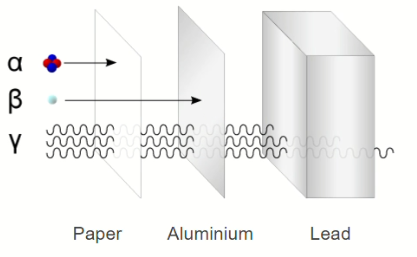
The amount of beta radiation absorbed depends on the thickness of the materials. This property of beta radiation is used to monitor the thickness of aluminium foil produced in an aluminium factory.
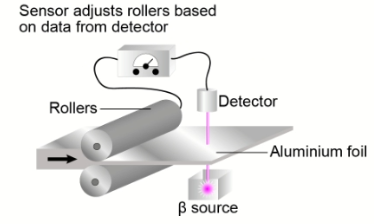
Question a (2 mark)
Your task is to design an investigation to determine how the thickness of aluminium foil affects the amount of beta radiation absorbed.
State the independent and dependent variables in your investigation.
Independent variable……
Dependent variable……..
▶️Answer/Explanation
Ans:
Independent variable: Thickness of the aluminium foil.
Dependent variable: Amount of beta radiation absorbed.
Question b (2 mark)
Outline the nature of beta radiation.
▶️Answer/Explanation
Ans:
Nature of beta radiation:
Beta radiation consists of high-energy electrons (beta particles) or positrons (positively charged electrons) that are emitted from the nucleus of an atom during radioactive decay. These particles have a negative charge and are much smaller and faster than alpha particles. Beta radiation can penetrate matter more deeply than alpha particles but less deeply than gamma radiation. It can be deflected by electric and magnetic fields, and its intensity can be reduced by absorptive materials, such as aluminum.
Question c (2 mark)
Formulate a hypothesis that would be tested by your investigation using scientific knowledge and understanding.
▶️Answer/Explanation
Ans:
Hypothesis: As the thickness of the aluminium foil increases, the amount of beta radiation absorbed will also increase.
Justification: Based on scientific knowledge and understanding, beta radiation consists of high-energy electrons or positrons emitted from the nucleus of an atom. The penetration of beta radiation depends on the thickness of the material it passes through. It is expected that as the thickness of the aluminium foil increases, more beta radiation particles will be absorbed by the foil due to increased interactions with the foil’s atoms. Therefore, it can be hypothesized that the amount of beta radiation absorbed will increase as the thickness of the aluminium foil increases.
The following video shows how the absorption of radiation can be measured with a Geiger-Muller tube.
The Geiger-Muller tube is linked to a digital rate meter. The rate meter gives readings in counts per second, which is a measure of the number of ionization’s detected by the Geiger-Muller tube each second.
You are provided with the following equipment:
- A source of beta radiation. In this case strontium-90 will be used. The radioactive source is in a sealed lead-lined container and produces a beam of beta radiation.
- A number of sheets of aluminium foil, with a thickness of $0.04 \mathrm{~mm}$
- Standard laboratory equipment (metre rules, clamps and clamp stands, etc.).

Question d (6 mark)
Explain the method you would use to collect data to test your hypothesis from part (c).
▶️Answer/Explanation
Ans:
Method to collect data to test the hypothesis:
1. Set up the equipment: Place the Geiger-Muller tube connected to the digital rate meter in a stable position. Ensure that the beta radiation source (strontium-90) is positioned at a fixed distance from the Geiger-Muller tube.
2. Establish a baseline reading: Take an initial reading on the digital rate meter without any aluminium foil present between the radiation source and the Geiger-Muller tube. This reading represents the count rate of beta radiation without any absorption.
3. Measure and place the aluminium foil: Cut a sheet of aluminium foil with a thickness of 0.04 mm. Secure the foil in front of the radiation source, ensuring it completely covers the area through which the radiation passes. Use clamps and clamp stands to hold the foil in place.
4. Take readings: Record the count rate displayed on the digital rate meter for a specific duration, such as 1 minute. This represents the count rate of beta radiation with the aluminium foil of 0.04 mm thickness absorbing some of the radiation.
5. Repeat the process: Remove the aluminium foil and take a second baseline reading. Then, repeat steps 3 and 4 for additional sheets of aluminium foil (increasing the thickness) to obtain data points for different foil thicknesses.
6. Analyze the data: Plot a graph with the thickness of the aluminium foil (independent variable) on the x-axis and the count rate of beta radiation (dependent variable) on the y-axis. Observe the trend in the data and evaluate whether the count rate increases as the thickness of the foil increases.
By following this method, data can be collected to test the hypothesis by measuring the effect of different thicknesses of aluminium foil on the absorption of beta radiation.
Question e (1 mark)
Background radiation is all around us. Background radiation does not come from the source; it is naturally occurring.
State one origin of the background radiation.
▶️Answer/Explanation
Ans:
Origin of background radiation: Cosmic radiation
Question f (2 mark)
Describe how the data collected could be corrected for the possible effects of background radiation.
▶️Answer/Explanation
Ans:
To correct the data for the possible effects of background radiation, the following approach can be used:
1. Take initial readings: Before starting the experiment, take baseline readings of the count rate on the digital rate meter without the presence of the beta radiation source or any aluminium foil. This provides a measure of the background radiation present.
2. Subtract background radiation: For each data point obtained during the experiment (with different thicknesses of aluminium foil), subtract the corresponding baseline reading (background radiation count rate) from the measured count rate. This correction accounts for the contribution of background radiation.
By subtracting the baseline readings from the measured count rates, the effects of background radiation can be accounted for, allowing for more accurate analysis and comparison of the count rates associated with the different thicknesses of aluminium foil.
Question g (4 mark)
State and justify two precautions you would take to ensure that the method was carried out safely.
▶️Answer/Explanation
Ans:
Precaution 1: Use appropriate shielding for radioactive sources.
Justification: Radioactive sources, such as the strontium-90 used in this experiment, emit ionizing radiation that can be harmful to living organisms if not properly shielded. It is essential to enclose the radioactive source in a sealed lead-lined container to prevent the direct exposure of individuals to the radiation. The lead lining effectively absorbs the radiation and minimizes the risk of exposure, ensuring the safety of the experimenters and others in the vicinity.
Precaution 2: Wear appropriate personal protective equipment (PPE).
Justification: Personal protective equipment should be worn to minimize the potential exposure to radiation during the experiment. This includes wearing lab coats, gloves, and safety goggles. Lab coats provide an additional barrier between the radioactive source and the experimenter’s skin, reducing the risk of direct contact. Gloves protect the hands from potential contamination and reduce the risk of radioactive material transferring to other surfaces. Safety goggles protect the eyes from any potential splashes or airborne particles. Wearing appropriate PPE ensures the safety of the experimenters and prevents unnecessary exposure to radiation.
These precautions help to ensure that the experiment is carried out safely by minimizing the risks associated with working with radioactive sources and reducing the potential exposure of individuals to ionizing radiation.
Question h (2 mark)
Imagine that you perform exactly the same experimental method as the one you planned but this time, you use an alpha source instead of the beta source.
Formulate a hypothesis for this second experiment using scientific knowledge and understanding in your answer.
▶️Answer/Explanation
Ans:
Hypothesis: As the thickness of the aluminium foil increases, the amount of alpha radiation absorbed will also increase.
Justification: Alpha particles consist of two protons and two neutrons, making them relatively large and heavy compared to beta particles. Due to their size and charge, alpha particles have limited penetration power and are easily absorbed by matter. It is expected that as the thickness of the aluminium foil increases, more alpha particles will interact with the foil’s atoms, leading to increased absorption. Therefore, it can be hypothesized that the amount of alpha radiation absorbed will increase as the thickness of the aluminium foil increases.
Question (12 marks)
We have seen that strontium- 90 has useful applications in industry but uncontrolled release of strontium-90 into the environment has negative consequences.
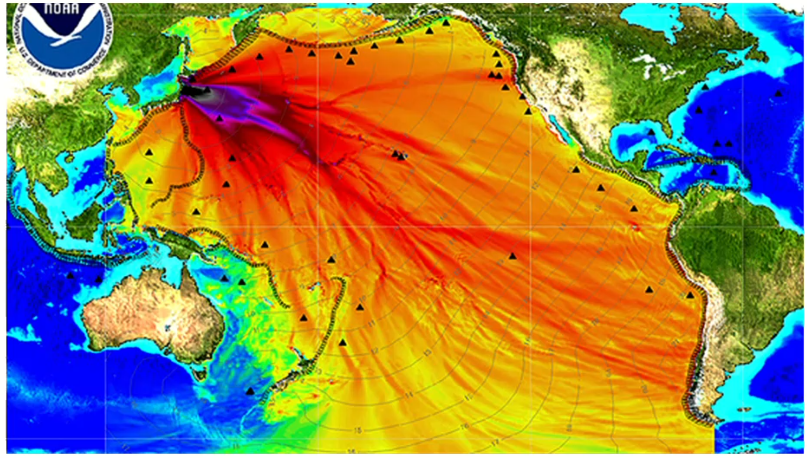
In 2011, there was a disaster at the Fukushima Daiichi nuclear power plant in Japan. This disaster resulted in the release of large amounts of radioactive water into the local environment; scientists think that this process is still occurring. The water contains many different radioisotopes including strontium-90. Some of this material made its way into the sea and has been spread globally as a result.
The map below shows a model of how the radioactive material released from Fukushima might spread across the world in seawater. The darker colour shows where the readings of radiation are predicted to be the highest.
The negative environmental effects of strontium-90 are clear, but to understand how long-lasting these effects will be, scientists must know the half-life of the radioisotope. The half-life is the time taken for the number of radioactive nuclei to decrease by half.
Radioactive decay is a random process. Strontium- 90 decays to yttrium- 90 by beta decay.
$$
{ }_{38}^{90} \mathrm{Sr} \rightarrow{ }_{39}^{90} \mathrm{Y}+{ }_{-1}^0 \beta
$$
Two years after the Fukushima Daiichi disaster, scientists wanted to investigate what was happening inside the reactor. Radiation levels inside the reactor were too high for humans to investigate directly so the scientists used a probe specially designed to work in radioactive conditions. The scientists found evidence of highly radioactive water leaking from the reactor.
Question a (6 mark)
The simulation below shows what could happen to a sample of 100 strontium- 90 nuclei over a period of 100 years.
You are going to collect data to enable you to plot a decay curve. You need to know how the number of strontium-90 nuclei varies with time. Time is your independent variable and number of strontium-90 nuclei is your dependent variable.
The table on the left below shows the data that you will record during the simulation.
Identify six times at which you will record data. Run and pause the simulation to collect this data.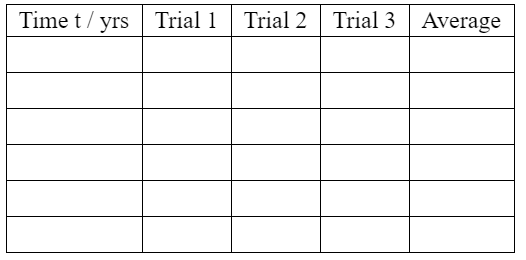
Calculate mean averages and enter them in the column headed “Average”. You should give your values to an appropriate number of significant figures.
▶️Answer/Explanation
Ans:
Another radioisotope that was released in the Fukushima Daiichi nuclear disaster was caesium-137. Another student used a simulation to collect decay data for caesium-137. Their data is presented in the graph below where time is on the $x$-axis and average percentage of caesium-137 remaining is on the y-axis.
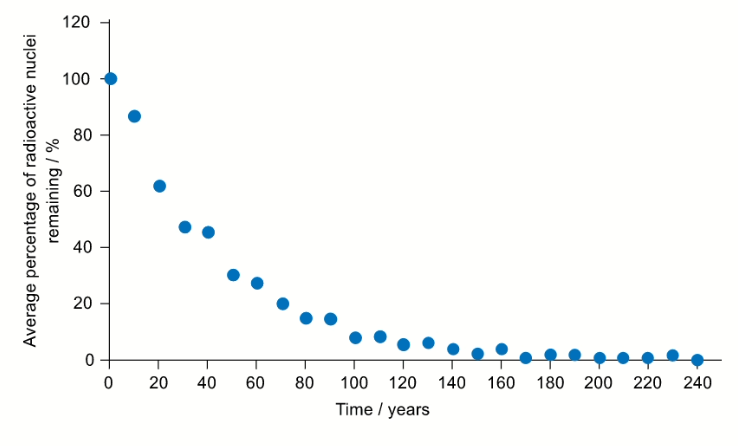
Question b (1 mark)
Some of the data is also presented in the graphs below. Click on the tabs to view the different graphs.
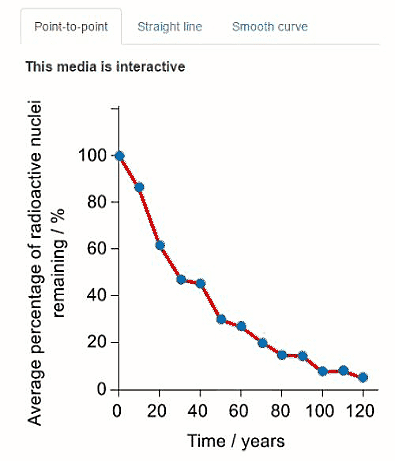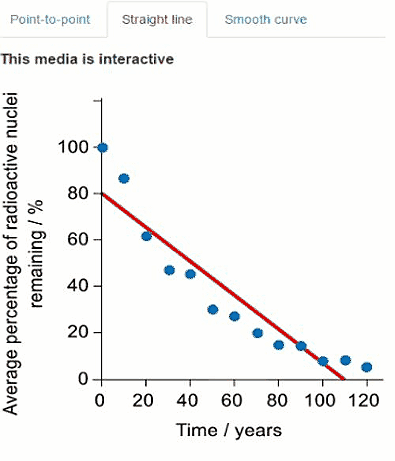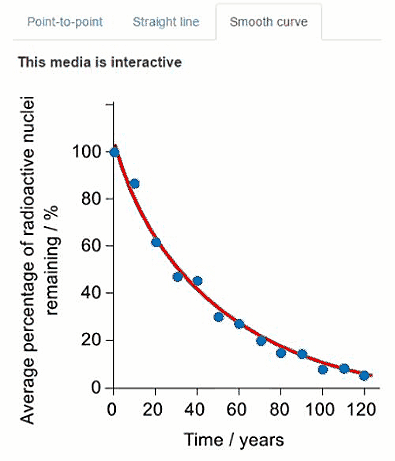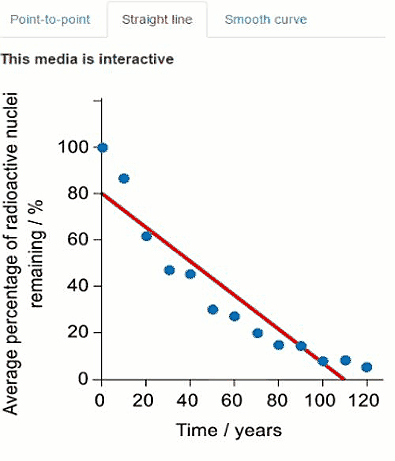
Select the most appropriate graph to present the data.
▶️Answer/Explanation
Ans:
Question c (3 mark)
The half-life is characteristic of a particular isotope.
Using the graph, calculate the average half-life for caesium-137. You should use at least three data points in your calculation.
▶️Answer/Explanation
Ans:
Question d (2 mark)
A different isotope of caesium, caesium-134 was also released. Caesium-134 has a half-life of two years.
If the Fukushima Daiichi disaster released 1600000 atoms of caesium-134 into the ocean, calculate how long it would take for this number to decrease to 100000 .
▶️Answer/Explanation
Ans:
Question (4 marks)
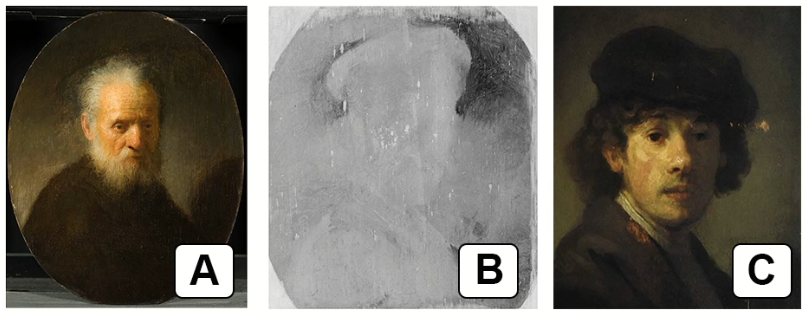
Compare image C to images A and B.
Some art historians suggest that Rembrandt reused the canvas shown in image B.
Question a (2 mark)
Outline the evidence in these three images that supports the suggestion that the canvas was reused.
▶️Answer/Explanation
Ans:
Evidence supporting the suggestion that the canvas was reused in Image B can be outlined by comparing it to Images A and C. Here are two marks of evidence:
1. Visible ghosting or underlying image: In Image B (x-ray fluorescence spectroscopy), there is a faint but discernible outline of a different painting visible beneath the surface layer. This indicates that the canvas had previously been used for another artwork before being painted over with the Old Man With A Beard. The presence of this underlying image suggests that the canvas was indeed reused by Rembrandt.
2. Differences in subject matter: Comparing the subject matter of the two paintings depicted in Images B and C, we can see that they are distinct. Image B shows the Old Man With A Beard, while Image C portrays a self-portrait of Rembrandt as a young man. The variation in subject matter further supports the idea that the canvas used for Image B was repurposed by Rembrandt for a new painting.
By considering these two pieces of evidence, the visible ghosting of a previous image and the difference in subject matter between the paintings, art historians can make a case for the reuse of the canvas in Image B.
Question b (2 mark)
Suggest two benefits of using XRFS to examine paintings rather than removing areas of paint.
▶️Answer/Explanation
Ans:
Using X-ray fluorescence spectroscopy (XRFS) to examine paintings instead of removing areas of paint offers several benefits. Here are two key advantages:
1. Non-destructive analysis: XRFS is a non-destructive technique that allows art historians and conservators to analyze the composition of a painting without physically removing any paint layers. Traditional methods, such as taking samples from the artwork, can be invasive and potentially damage or alter the artwork. By using XRFS, the integrity and original state of the painting can be preserved, minimizing the risk of irreversible damage.
2. Revealing hidden information: XRFS can provide valuable insights into the materials and techniques used by artists, as well as uncover hidden information about the history of a painting. The technique can detect and map the distribution of various elements within the layers of paint, allowing researchers to identify pigments, analyze layer structures, and even detect hidden or underdrawn sketches or compositions. This non-invasive approach enables art historians to gather information about an artwork’s creation process, artistic choices, and possible alterations without physically altering the painting.
Overall, the use of XRFS in examining paintings offers the benefits of non-destructive analysis and the potential for uncovering hidden information, allowing for a deeper understanding of the artwork while preserving its original condition.
Question (23 marks)
X-rays are a form of ionising radiation. Other forms of ionising radiation include alpha particles, beta particles and gamma rays.
Question a (3 mark)
Describe the process of ionisation by one of the forms of ionising radiation.
▶️Answer/Explanation
Ans:
One form of ionizing radiation is alpha particles. Alpha particles are composed of two protons and two neutrons, which are essentially the same as the nucleus of a helium atom. The process of ionization by alpha particles occurs when these particles interact with atoms or molecules in a material.
When an alpha particle approaches an atom, it exerts a strong electromagnetic force on the electrons in the atom’s outer shells. The strong positive charge of the alpha particle attracts the negatively charged electrons. As the alpha particle passes close to the atom, it can strip away one or more electrons from the atom’s outer shell.
This removal of electrons from the atom results in the creation of positively charged ions. The atom, now missing one or more electrons, becomes positively charged. The freed electrons, in turn, can interact with other atoms or molecules, causing additional ionization. This cascading effect can lead to the creation of a trail of ionized atoms and molecules along the path of the alpha particle.
The process of ionization by alpha particles is highly localized, as the particles are relatively massive and tend to interact strongly with matter. Due to their size and charge, alpha particles have a limited range and can be stopped by a few centimeters of air or a thin layer of material. This characteristic makes alpha particles less penetrating compared to other forms of ionizing radiation like beta particles and gamma rays.
Question b (2 mark)
Outline the danger of ionising radiation for living cells.
▶️Answer/Explanation
Ans:
Ionizing radiation can pose dangers to living cells due to its ability to ionize atoms and molecules within the cells. Here are two main dangers associated with ionizing radiation:
1. DNA Damage: Ionizing radiation has enough energy to directly or indirectly damage the DNA within cells. When ionizing radiation passes through a cell, it can cause ionization of atoms and molecules, leading to the formation of free radicals. These highly reactive and unstable species can interact with the DNA molecule, causing breaks, cross-links, or alterations in the DNA structure. Severe DNA damage can disrupt the cell’s ability to properly replicate, repair, or regulate its genetic material, which can result in mutations, genetic instability, or cell death.
2. Cell Function Impairment: Ionizing radiation can also damage other vital cellular components, such as proteins, membranes, and organelles. The ionization process can disrupt the normal functioning of these cellular structures, leading to dysfunction and impairment of essential cellular processes. This can interfere with cellular metabolism, energy production, and signal transduction pathways, ultimately affecting the overall health and viability of the cell.
The cumulative effects of DNA damage and cellular dysfunction caused by ionizing radiation can have various consequences, including cell death, impaired tissue function, or the development of radiation-related diseases such as cancer. The severity of the danger depends on the type and dose of radiation, as well as the sensitivity and resilience of the specific cell or tissue type exposed to it. Proper protection and safety measures are necessary to minimize the risks associated with ionizing radiation exposure.
Question c (1 mark)
X-rays, gamma rays and ultraviolet light are all forms of electromagnetic radiation.
Label the diagram of the electromagnetic spectrum.
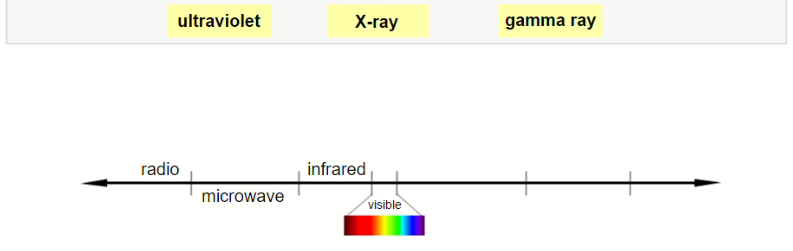
▶️Answer/Explanation
Ans:
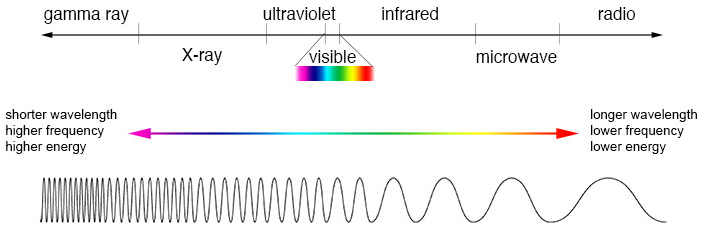
Question d (3 mark)
X-rays and gamma rays can both be used by doctors to produce images of the internal structure of the human body. The different properties of X-rays and gamma rays produce different types of image.
An X-ray image is formed by projecting X-rays, and then capturing the “shadow” on a surface that reacts to X-ray radiation.
Using information from the table, discuss why X-rays are used, rather than ultraviolet or gamma rays, when doctors wish to make images of a person’s bones.
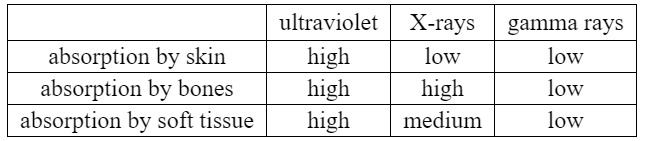
▶️Answer/Explanation
Ans:
When doctors wish to make images of a person’s bones, X-rays are used rather than ultraviolet or gamma rays. This choice is based on the differential absorption properties of these types of radiation as indicated in the table.
X-rays have a relatively low absorption by skin, allowing them to penetrate through the body’s outer layers and reach the underlying bones. This characteristic is advantageous for imaging purposes since it enables X-rays to pass through the soft tissues and reach the bones, which have a relatively high absorption of X-rays. As a result, the bones appear as contrasting structures on the X-ray image, while the surrounding soft tissues and skin contribute less to the overall image formation.
On the other hand, ultraviolet rays have high absorption by both the skin and bones. Their high absorption by the skin limits their penetration depth, making it difficult for them to reach the bones effectively. This characteristic makes ultraviolet rays less suitable for bone imaging purposes.
Similarly, gamma rays have low absorption by both bones and soft tissues. While this property allows gamma rays to penetrate through the body, it also means that they are less likely to be absorbed or attenuated by the bones. As a result, gamma rays do not produce distinct contrast between bones and surrounding tissues, making them less effective for bone imaging compared to X-rays.
In summary, X-rays are used for imaging bones because they have relatively low absorption by skin, allowing them to penetrate through the body and reach the bones, which have a higher absorption of X-rays. This differential absorption property of X-rays enables the production of clear and contrasting images of the bones while minimizing the contribution of surrounding soft tissues and skin.
Question e (14 mark)
X-rays are not the only means of producing medical images. There are different options for producing medical images. Information about some of the options is presented in the tables below.
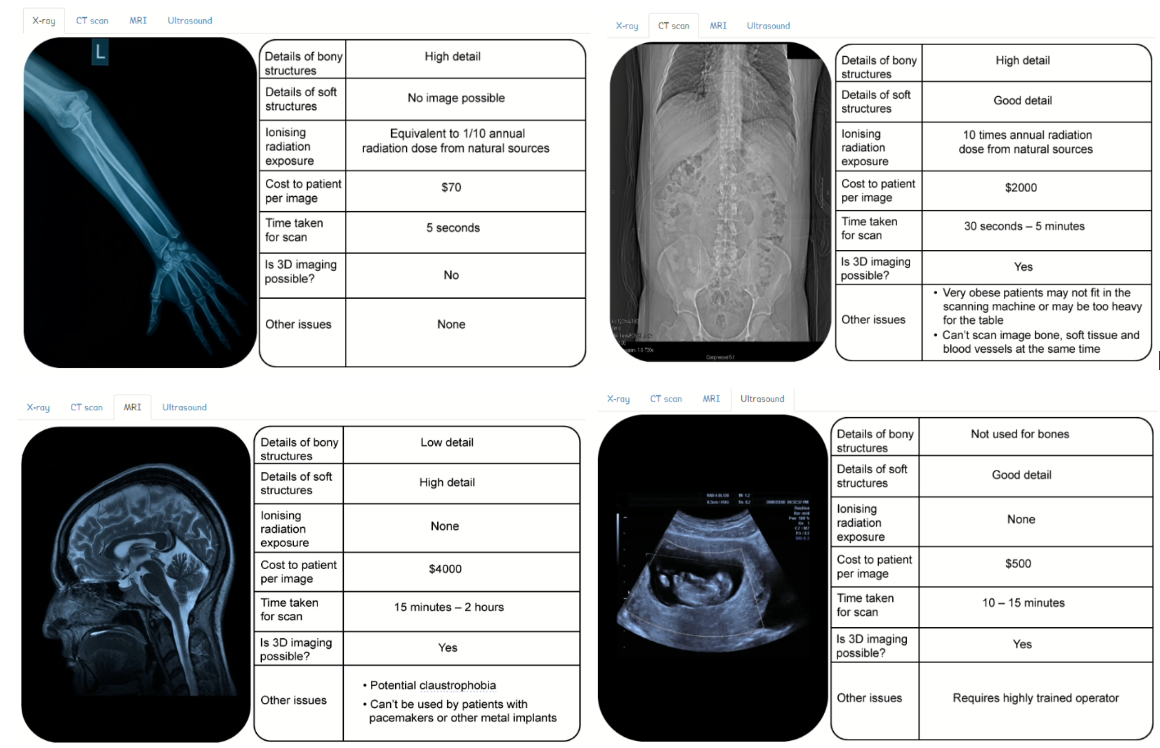
All hospitals have a limited amount of money to spend on medical equipment. Hospital managers have to balance the advantages and disadvantages of different types of equipment when they decide how to spend their money.
Using the information in the tables, discuss and evaluate the medical imaging equipment you would recommend to the hospital manager, clearly justifying your recommendation. In this extended piece of writing, you should consider the social and economic factors and include:
- the advantages of your chosen equipment
- the disadvantages of your chosen equipment
- the perspective of the hospital
- the perspective of the patients.
▶️Answer/Explanation
Ans:
To provide a comprehensive evaluation and recommendation for medical imaging equipment, let’s consider the information from the tables and the perspectives of both the hospital and the patients. The tables provide information on four different types of medical imaging options: X-ray, computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound.
1. X-ray Imaging:
Advantages:
- Cost-effective: X-ray equipment is generally more affordable compared to other imaging modalities, making it a suitable choice for hospitals with limited budgets.
- Quick imaging: X-rays provide rapid results, allowing for efficient diagnosis and treatment decisions.
- Wide availability: X-ray technology is widely accessible and commonly used in hospitals, ensuring familiarity and ease of use for medical professionals.
Disadvantages:
- Limited tissue differentiation: X-rays primarily highlight differences in tissue density, which means they may not provide detailed information on soft tissues or subtle abnormalities.
- Ionizing radiation exposure: X-rays involve ionizing radiation, which carries a potential risk of cumulative radiation exposure over time. Steps must be taken to minimize patient radiation dose.
Hospital Perspective:
X-ray imaging is a fundamental and cost-effective imaging modality that can provide valuable information for a wide range of medical conditions. It is especially useful for bone imaging and initial assessments. Considering the limited budget, the hospital can allocate funds to maintain and upgrade X-ray equipment while ensuring proper radiation safety protocols.
Patient Perspective:
From the patient’s viewpoint, X-ray imaging offers advantages such as quick results, relatively low cost, and non-invasive nature (as it does not involve injections or invasive procedures). However, patients may have concerns about radiation exposure. It is essential for the hospital to prioritize patient safety, implement appropriate dose optimization techniques, and educate patients about the benefits and risks of X-ray imaging.
2. Computed Tomography (CT):
Advantages:
- Rapid and detailed imaging: CT scans provide detailed cross-sectional images, allowing for accurate diagnosis and treatment planning.
- Wide range of applications: CT is useful for imaging various body regions, including the chest, abdomen, and pelvis.
- Advanced imaging capabilities: Modern CT scanners can perform advanced techniques such as angiography and 3D reconstruction.
Disadvantages:
- Ionizing radiation exposure: CT uses ionizing radiation, which carries a higher radiation dose compared to other modalities, posing potential health risks.
- Cost and space requirements: CT equipment and maintenance are costly, and a dedicated space with radiation shielding is necessary.
Hospital Perspective:
CT imaging plays a crucial role in emergency medicine, trauma assessment, and detailed anatomical evaluation. However, due to the high initial and operational costs, the hospital must carefully consider the patient population’s needs and allocate resources accordingly.
Patient Perspective:
CT scans provide rapid and detailed imaging, enabling timely diagnosis and treatment. However, patients may have concerns about radiation exposure. Hospitals should prioritize radiation dose optimization, follow appropriate imaging protocols, and communicate the benefits and risks to patients.
3. Magnetic Resonance Imaging (MRI):
Advantages:
- Excellent soft tissue contrast: MRI provides superior soft tissue visualization, making it ideal for evaluating the brain, spinal cord, joints, and soft organs.
- Multi-planar imaging: MRI can acquire images in multiple planes, facilitating comprehensive anatomical assessment.
- No ionizing radiation: MRI does not involve ionizing radiation, eliminating radiation exposure concerns.
Disadvantages:
- High cost: MRI equipment and maintenance are expensive, making it a significant investment for hospitals.
- Patient limitations: Certain patients with claustrophobia, pacemakers, or metal implants may face challenges in undergoing an MRI examination.
Hospital Perspective:
MRI is a valuable imaging modality for detailed anatomical assessment and characterization of various pathologies. However, the high cost of MRI equipment and the need for dedicated space and specialized technical expertise should be considered in the budget allocation.
Patient Perspective:
Patients appreciate the superior image quality and the absence of radiation exposure in MRI. However, the lengthy scanning time and potential claustrophobia can be sources of anxiety for some patients. Hospitals should prioritize patient comfort by offering open MRI options, ensuring clear communication, and providing necessary support during the examination.
4. Ultrasound Imaging:
Advantages:
- Non-ionizing radiation: Ultrasound uses sound waves instead of ionizing radiation, making it a safe option for repeated imaging and monitoring during pregnancy or pediatric cases.
- Real-time imaging: Ultrasound provides real-time imaging, allowing dynamic visualization of structures and functions.
- Portable and versatile: Ultrasound machines are typically portable and can be used at the bedside or in various clinical settings, enhancing accessibility and flexibility.
Disadvantages:
- Operator-dependent: Obtaining high-quality ultrasound images requires skilled operators, and the interpretation of images can be subjective.
- Limited penetration: Ultrasound may have limitations in imaging deep-seated structures or patients with obesity or gas-filled intestines.
Hospital Perspective:
Ultrasound imaging offers versatility and flexibility for various clinical scenarios, including obstetrics, cardiology, and point-of-care assessments. The hospital can consider investing in advanced ultrasound systems with additional features and training opportunities for operators to maximize the benefits of this modality.
Patient Perspective:
Patients often appreciate the non-invasive nature of ultrasound imaging, absence of radiation exposure, and the ability to visualize real-time images. Ultrasound is commonly used in prenatal care and can provide reassurance for patients during the imaging process.
In conclusion, considering the social and economic factors, the hospital manager should prioritize allocating funds to maintain and upgrade the existing X-ray equipment due to its cost-effectiveness, wide availability, and usefulness in bone imaging. Simultaneously, investments in advanced ultrasound systems and operator training can enhance imaging capabilities and expand its utility in various clinical scenarios. While MRI and CT offer superior imaging capabilities, their high costs and specific patient requirements warrant careful consideration and budget allocation based on the hospital’s specific needs and patient population. Ultimately, a balanced approach that considers both the hospital’s financial constraints and the patients’ needs for safe and accurate diagnosis should guide the equipment recommendation.
Question (3 marks)
Look at the two very different images of feet.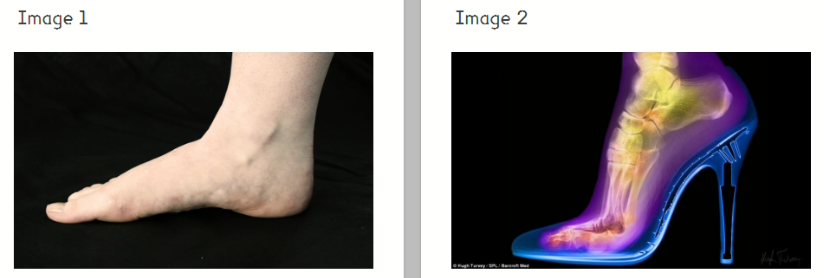
The second image is by the artist Hugh Turvey who uses X-rays in his work. This piece of art is called Femme Fatale and shows the foot of a woman wearing a high-heeled shoe. The artist has used an X-ray, normally used in science, medicine or industry to create this artistic image.
Outline what the use of science can reveal that a photograph does not. Refer to the image and apply the ideas that you have been introduced to in this task in your answer.
▶️Answer/Explanation
Ans:
The use of science, specifically X-ray imaging, in creating the artistic image by Hugh Turvey can reveal certain aspects that a traditional photograph may not capture. Here are a few points to consider:
1. Internal Structure: X-ray imaging allows us to see beyond the external appearance of an object or subject. In the case of the X-ray image of the foot in Femme Fatale, it reveals the internal structure of the foot, showcasing the skeletal framework and the positioning of bones. This provides a unique perspective and adds a layer of depth to the image that a photograph cannot convey.
2. Transparency: X-rays have the ability to penetrate certain materials, such as soft tissues, and showcase their level of transparency. In the image, the X-ray reveals the transparency of the shoe, allowing us to see through the material and observe the underlying foot structure. This transparency aspect adds an intriguing and somewhat surreal quality to the artwork, providing a different aesthetic experience compared to a traditional photograph.
3. Hidden Details: X-ray imaging can unveil hidden or obscured details that are not visible in a photograph. It can highlight subtle nuances or features that are not easily perceivable through conventional photography. In the case of Femme Fatale, the X-ray image exposes the intricate details of the foot’s bone structure and the alignment of joints, presenting an anatomical perspective that is typically hidden from plain sight.
4. Scientific Context: The use of X-rays, a scientific technology primarily employed in fields such as medicine and industry, brings a scientific context to the artwork. It bridges the gap between art and science, creating an intriguing juxtaposition that stimulates thought and reflection on the intersection of these disciplines.
Overall, by utilizing X-ray imaging in the creation of the artistic image, the artist, Hugh Turvey, offers a unique perspective that goes beyond what a photograph can convey. The scientific application of X-rays reveals the internal structure, transparency, hidden details, and scientific context, enhancing the aesthetic and conceptual dimensions of the artwork.
Question:
Red light has a wavelength around 650nm, the wavelength of yellow is about 570nm and blue is about 475nm. A certain color of light has a frequency of 5.66 × 1014Hz. What color is this light?
▶️Answer/Explanation
Ans: \(\lambda =\frac{3\times 10^{8}}{5.66\times 10^{14}}=530 nm\)
This is between yellow and blue so the light is green.
Question:
A remote control uses light at 940nm.
a) In which part of the electromagnetic spectrum does this lie?
▶️Answer/Explanation
Ans: Infrared
b) Calculate the frequency of the light.
▶️Answer/Explanation
Ans: \(\frac{3\times 10^{8}}{940\times 10^{-9}}=3.19\times 10^{14}Hz\)
Question:
Photons of light emitted from a nucleus of \(_{28}^{60}\textrm{Ni}\) have a frequency of 3.2 × 1020Hz.
a) In which region of the electromagnetic spectrum is this radiation classed?
▶️Answer/Explanation
Ans: Gamma
b) Calculate the wavelength of these photons.
▶️Answer/Explanation
Ans: \(\frac{3\times 10^{8}}{3.2\times 10^{20}}=9.38\times 10^{-13}Hz\)
Question:
A radio signal is broadcast at 200 kHz. Calculate the wavelength of this broadcast. (Remember that all electromagnetic waves travel at the speed of light.)
▶️Answer/Explanation
Ans: \(\frac{3\times 10^{8}}{200\times 10^{3}}=1500 m\)
Question:
Mobile phones which operate on a 4G system use wavelengths of 800MHz, 1.8GHz and 2.6GHz.
a) Which of these has the smallest wavelength?
▶️Answer/Explanation
Ans: 2.6 GHz
b) Express 800MHz in GHz.
▶️Answer/Explanation
Ans: 0.8 GHz (1 GHz = 1000 MHz)
c) Calculate the wavelength and time period of the 2.6GHz frequency.
▶️Answer/Explanation
Ans: \(\lambda =\frac{3\times 10^{8}}{2.6\times 10^{9}}=0.12 m;\)
\(T =\frac{1}{2.6\times 10^{9}}=3.9\times 10^{-10}s\)
Question:
UVA light has wavelengths of 315–400nm and UVB has wavelengths of 280–315nm.
a) Which of these regions is closer to visible light?
▶️Answer/Explanation
Ans: Visible light has wavelengths 400 – 700 nm so UVA
b) In which UV range does light with a frequency of 1015Hz lie?
▶️Answer/Explanation
Ans: \(\frac{3\times 10^{8}}{10^{15}}=300 nm so UVB\)
Question:
If soft X-rays have wavelengths of 10–0.1nm and hard X-rays have wavelengths less than 0.1nm, what type of X-ray has a frequency of 3 × 1017Hz?
▶️Answer/Explanation
Ans: \(\lambda =\frac{3\times 10^{8}}{3\times 10^{17}}=10^{-9}m=1 nm; a soft X-ray.\)
Question:
A force of 10N is an enormous force to be exerted on a proton which has a mass of 1.67 × 10–27kg.
a) Using the equation F = ma, calculate the acceleration of a proton which experiences a 10N force.
▶️Answer/Explanation
Ans: \(\frac{10}{1.67\times 10^{-27}}=5.99\times 10^{27}s\)
b) The size of an atomic nucleus is about 10–15m. If the proton is accelerated across this distance by a 10N force, use the equation W = Fd to calculate the work done by the force.
▶️Answer/Explanation
Ans: 10 × 10–15 = 10–14 J
c) As this work is transferred to kinetic energy, calculate the final speed of the proton.
▶️Answer/Explanation
Ans: \(\sqrt{\frac{2\times 10^{-14}}{1.67\times 10^{-27}}}=3.46\times 10^{6} ms^{-1}\)
d) Why does the electrostatic repulsion of protons in the nucleus not affect a nucleus of hydrogen?
▶️Answer/Explanation
Ans: A hydrogen nucleus has only one proton.
Question:
Astatine is the rarest naturally occurring element on Earth. Nuclei of its most stable isotope \(_{85}^{210}\textrm{At}\) only last an average of 12hours before they decay by alpha decay. What does \(_{85}^{210}\textrm{At}\) decay into? You may need a periodic table to finnd which element is formed.
▶️Answer/Explanation
Ans: \(_{85}^{210}\textrm{At}\rightarrow _{2}^{4}\textrm{}\alpha \rightarrow _{83}^{206}\textrm{Bi}\)
Question:
Radon gas \(\left ( _{86}^{222}\textrm{Rn} \right )\) is formed when uranium-234 \(\left ( _{92}^{234}\textrm{U} \right )\) decays by a series of alpha decays. How many alpha decays are needed for uranium-234 to decay to radon-222?
▶️Answer/Explanation
Ans: 234 = 4x + 222; x = 3
Question:
Nitrogen-16 \(\left ( _{7}^{16}\textrm{N} \right )\) is an isotope of nitrogen with two more neutrons than the more common nitrogen-14. It decays through beta decay. What will it decay into? (You may need a periodic table to find which element it decays into.)
▶️Answer/Explanation
Ans: \(_{7}^{16}\textrm{N}\rightarrow _{-1}^{0}\textrm{}\beta \rightarrow _{8}^{16}\textrm{O}\)
Question:
Iodine-135 \(\left ( _{53}^{135}\textrm{I} \right )\) is created in nuclear power plants. It decays into barium-135 \(\left ( _{56}^{135}\textrm{Ba} \right )\) through a series of beta decays. How many beta decays must occur?
▶️Answer/Explanation
Ans: 53 = 56 – x; x = 3
Question:
Why is it important to design a Geiger–Müller tube to have a thin front window if it is to be used to detect alpha radiation?
▶️Answer/Explanation
Ans: Alpha particles can only travel a couple of centimeters through air so the window would block them if it were too thick.
Question:
The activity of a radioactive sample is tested with a Geiger–Müller tube. The number of counts in one minute is measured three times and found to be 277, 251 and 282. If the Geiger–Müller tube detects 25% of the radiation emitted, calculate the measured activity.
▶️Answer/Explanation
Ans: Average counts per minute = \(\frac{277+251+282}{3}=270; activity =\frac{270\times 4}{60}=18 Bq\)
Question:
At 9.00a.m. on a Monday, the activity of a sample of sodium-24 is measured to be 2,400Bq. By midday the following Thursday, the activity has fallen to 75Bq. What is the half-life of sodium-24?
▶️Answer/Explanation
Ans: \(\frac{2400}{75}=32;\)
25 = 32 so the activity of the sample has halved 5 times; time elapsed = 75 hrs; \(half-life=\frac{75}{5}=15 hrs\)
Question:
A sample of uranium-240 has an activity of 20,480Bq. After one week it has decayed until its activity is 5Bq. What is its half-life?
▶️Answer/Explanation
Ans: \(\frac{20480}{5}=4096;\)
212 = 4,096 so the activity of the sample has halved 12 times; \(half-life=\frac{168}{12}=12 hrs\)
Question:
Oganesson-294 \(\left ( _{118}^{294}\textrm{Og} \right )\) was first synthesized in 2002. It has a half-life of 0.7ms. What is the probability of an atom of oganesson lasting for more than 3.5ms?
▶️Answer/Explanation
Ans: 0.55 = 0.03125
Question:
The equation for the formation of carbon-14 when nitrogen absorbs a neutron in the atmosphere is:
\(_{7}^{14}\textrm{N}+_{0}^{1}\textrm{n}\rightarrow _{6}^{14}\textrm{C}+_{?}^{?}\textrm{X}\)
By considering what the numbers represented by question marks must be, deduce what particle X is.
▶️Answer/Explanation
Ans: A proton
Question:
An early human settlement is discovered and archeologists recover a stone axe, some animal bones, and some burnt wood from a fire.
a) Which of these could be dated using carbon dating?
▶️Answer/Explanation
Ans: Animal bones and burnt wood as they will contain carbon which was sourced at the time of the settlement.
b) A sample of material from the settlement contains one-fifth of the 14C proportion that a modern sample would have. How old does this suggest that the settlement is?
▶️Answer/Explanation
Ans: From graph: 13,250 years (±250)
Question:
Why is it not possible to use carbon dating to determine the age of a dinosaur bone from 65 million years ago?
▶️Answer/Explanation
Ans: The half-life of carbon–14 is too short. There would not be enough difference in proportion of carbon–14 to accurately determine the age of the bones.
Question:
Use a periodic table to work out what element the cesium isotopes decay into.
▶️Answer/Explanation
Ans: \(_{55}^{134}\textrm{Cs}\rightarrow _{-1}^{0}\textrm{}\beta +_{56}^{134}\textrm{Ba}\)
\(_{55}^{135}\textrm{Cs}\rightarrow _{-1}^{0}\textrm{}\beta +_{56}^{135}\textrm{Ba}\)
Barium
Summative assessment
The dangers of nuclear and electromagnetic radiation
Question:
Ultraviolet radiation can be dangerous to humans. Much of the UV light from the Sun is blocked by the atmosphere.
a) State the name of the chemical in the atmosphere which blocks dangerous UV light.
▶️Answer/Explanation
Ans: Ozone
b) What are the dangers of UV light and how can they be avoided?
▶️Answer/Explanation
Ans: One mark for each danger and one for each way of avoiding:
Skin cancer; can be avoided by wearing sun cream and limiting sun exposure.
Cataracts; can be avoided by wearing sunglasses.
Question:
Nuclear radiation can also be dangerous.
a) Give an example of the dangers of nuclear radiation.
▶️Answer/Explanation
Ans: Ionizing properties of alpha and beta radiation can cause DNA to mutate; which can lead to cancer.
b) Suggest a sensible safety precaution when handling radioactive sources.
▶️Answer/Explanation
Ans: Valid safety precaution which reduces exposure. For example:
- Exposure should be kept to a minimum;
- Direct contact should not be made (i.e. wear gloves, protective suit, handle with tongs).
Question:
Dangerous radiation is often called ionizing radiation.
a) What is meant by ionizing?
▶️Answer/Explanation
Ans: Removes electrons from the material it passes through; leaving it ionized.
b) Which parts of the electromagnetic spectrum are ionizing?
▶️Answer/Explanation
Ans: Gamma rays; X-rays.
c) Which type of nuclear radiation is the most ionizing?
▶️Answer/Explanation
Ans: Alpha radiation
d) How far through air would you expect nuclear radiation to travel?
▶️Answer/Explanation
Ans: One mark for one correct, two for all three correct.
- Alpha: a few centimeters
- Beta: a few meters
- Gamma: a few kilometers
Investigating beta radiation
A class experiment uses a radioactive source to investigate how far beta radiation travels through air. A detector is positioned at varying distances from the radioactive source and the number of counts in a period of 1minute is detected.
Question:
Identify the independent and dependent variables for this experiment.
▶️Answer/Explanation
Ans: Independent variable – distance from source; Dependent variable – number of counts in 1 minute.
Question:
Suggest a suitable detector for this experiment.
▶️Answer/Explanation
Ans: Geiger–Müller tube
Question:
Suggest a suitable set of distances that could be investigated in the experiment.
▶️Answer/Explanation
Ans: More than 5 distances investigated; range should run from less than 1 m; to more than 3 m
Question:
There are suspicions that the radioactive source is emitting gamma rays as well as beta radiation. Explain how a thin piece of metal can help to distinguish how much of the detected radiation
is gamma and how much is beta.
▶️Answer/Explanation
Ans: Beta radiation would not be able to pass through piece of metal; gamma would be able to pass through metal; when the metal is placed in front of the detector, only gamma and background radiation is detected; compare background count with source shielded by metal so see if there is a difference.
Question:
It is important to take background radiation into account.
a) Suggest one possible source of background radiation.
▶️Answer/Explanation
Ans: One from:
- Radon gas from rocks
- Cosmic rays
- Food and drink
b) Explain how background radiation could be taken into account in this experiment.
▶️Answer/Explanation
Ans: 1. Remove source;
2. Measure count rate for 5 minutes;
3. Divide count rate by 5 to find the average count rate for 1 minute. This is the average background radiation count in 1 minute;
4. Subtract this value from each reading in the experiment.
Studying how soft X-rays pass through air
A student knows that soft X-rays are known to be blocked by air easily and that hard X-rays can travel long distances through air. She forms a hypothesis that the distance X-rays can travel is directly proportional to the frequency of the X-rays. In order to test her hypothesis, she tries to find some data. She discovers this graph in a scientific paper. It shows the percentage of X-rays which can travel a certain distance in air. The graph shows results for different wavelengths of X-rays.
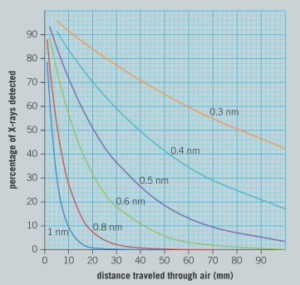
The student uses this data from this experiment to find the amount of air required to block half of the X-rays at different wavelengths.
Question:
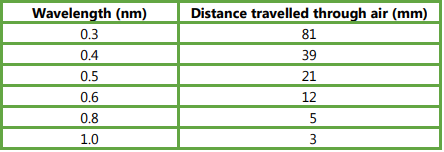
Read off values from the graph to find the distance the different wavelengths of X-rays travel before half are absorbed. Record your data in a suitable table.
▶️Answer/Explanation
Ans: Table of six values; correct values (see below, allow ±1 mm); column headings; with units.
Question:
Plot a graph of your data.
▶️Answer/Explanation
Ans: 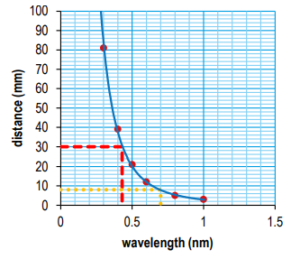
Question:
Add a line of best fit to your graph.
▶️Answer/Explanation
Ans: 
Question:
Describe the trend of your data.
▶️Answer/Explanation
Ans: Non-linear; as wavelength increases, distance travelled decreases at a decreasing rate.
Question:
Use your graph to find:
a) the wavelength of X-rays for which half would be absorbed by 30mm of air
▶️Answer/Explanation
Ans: 0.43 nm (allow ±0.01)
b) the distance that X-rays with a wavelength of 0.7nm could travel before half are absorbed.
▶️Answer/Explanation
Ans: 8 mm
Question:
The student’s original hypothesis was that the frequency of the X-rays is directly proportional to the distance they traveled. Suggest whether the hypothesis is supported or contradicted by the data.
▶️Answer/Explanation
Ans: Frequency is inversely proportional to wavelength; if wavelength is doubled, frequency is halved and hypothesis suggests distance is halved; values read from graph. For example, wavelengths of 0.4 and 0.8 mm have distances of 39 and 5 mm; hence doubling wavelength gives a 7.8 times reduction in distance; relationship between frequency and distance is not directly proportional; hypothesis is incorrect.
Question:
The student writes a report on her findings. Explain why is it important that she references the scientific paper in which she found the original graph.
▶️Answer/Explanation
Ans:
- Gives credit to author(s);
- Provides evidence to support her own work;
- Demonstrates that her sources are reliable.
Question:
The scientific paper from which the data came refers to the X-rays as radiation. Other pupils in her class thought that radiation referred to radioactive decay. Write a brief explanation of the
similarities and differences between these two types of radiation. Try to use scientific terms correctly.
▶️Answer/Explanation
Ans: 1 mark for each similarity (up to 2 marks); 1 mark for each difference (up to 2); 2 marks for correct use of scientific terminology. For example:
Similarities
- Both ionizing;
- Both transfer energy;
- Gamma rays and X rays are both high frequency electromagnetic rays.
Differences
- Radioactive decay originates from the nucleus of atoms; X rays are generated with high voltages;
- Alpha and beta emission carry mass;
- Alpha and beta emission carry charge;
- Alpha and beta emission have higher ionisation/shorter range.
Question:
X-rays of a similar wavelength can be used in astronomy. This is a picture of the Crab Nebula, the remnant of a supernova, taken using X-rays of frequencies between about 1 × 1017 and
2 × 1018Hz. It shows the neutron star at the center of the nebula. Explain why the X-ray telescope had to be in space, in orbit around the Earth, rather than on the ground.
▶️Answer/Explanation
Ans: X-rays are absorbed by the atmosphere; telescope has to be in space to observe enough X-ray intensity.
