IB MYP 4-5 Biology-Biochemistry and Enzymes- Study Notes - New Syllabus
IB MYP 4-5 Biology-Biochemistry and Enzymes- Study Notes – New syllabus
IB MYP 4-5 Biology-Biochemistry and Enzymes- Study Notes – IB MYP 4-5 Biology – per latest IB MYP Biology Syllabus.
Key Concepts:
- Enzyme structure and function
- Factors affecting enzyme activity (pH, temperature)
- Lock-and-key vs induced fit models
Enzyme Structure and Function
What Are Enzymes?
Enzymes are biological catalysts proteins that accelerate chemical reactions in living organisms without being used up. They are essential for processes such as digestion, respiration, and DNA replication.
Key Features of Enzymes
- Specific: Each enzyme acts on a specific substrate.
- Reusable: Not consumed or altered during the reaction.
- Efficient: Lower activation energy to speed up reactions.
- Sensitive: Affected by temperature, pH, and concentration.
Structure of Enzymes
Enzymes are made of long amino acid chains folded into a unique 3D shape. This shape determines their specific function. The active site is the most important region it binds the substrate.
Two Models of Enzyme Action
1. Lock and Key Model: The enzyme’s active site has a fixed shape that exactly fits the substrate.
2. Induced Fit Model: The active site slightly changes shape to fit the substrate more precisely.
How Enzymes Work (Mechanism)
- Substrate binds to the enzyme’s active site.
- Forms an enzyme-substrate complex.
- The enzyme catalyzes the reaction.
- Products are released; the enzyme is free to repeat the process.
Factors Affecting Enzyme Activity
| Factor | Effect on Enzyme Activity |
|---|---|
| Temperature | Activity increases up to an optimum (about 37°C in humans). Too high = denaturation. |
| pH | Each enzyme has an optimum pH. Outside this range = loss of function. |
| Substrate concentration | Rate increases until enzymes are saturated. |
| Enzyme concentration | More enzymes = faster reaction (if substrate is available). |
What Happens When Enzymes Denature?
Denaturation is when the enzyme’s 3D shape is changed by heat or pH, especially the active site. A denatured enzyme cannot function and this change is irreversible.
Examples of Important Enzymes in the Human Body
| Enzyme | Substrate | Product | Where Found |
|---|---|---|---|
| Amylase | Starch | Maltose | Saliva, small intestine |
| Pepsin | Proteins | Peptides | Stomach (acidic pH) |
| Lipase | Lipids | Fatty acids + glycerol | Pancreas, small intestine |
| Maltase | Maltose | Glucose | Small intestine |
Related Insight: Lactase and Lactose Intolerance
Lactase is an enzyme that breaks down lactose (milk sugar) into glucose and galactose. Some individuals produce less lactase after infancy, leading to lactose intolerance. This reflects an evolutionary adaptation in populations that do not consume milk regularly after weaning.
Summary
- Enzymes are proteins that speed up chemical reactions.
- They work by binding substrates at the active site.
- Enzyme structure determines their function.
- Temperature, pH, and concentration affect activity.
- Without enzymes, life-sustaining reactions would be too slow.
Factors Affecting Enzyme Activity (pH and Temperature)
Why Are Enzymes Sensitive to Conditions?
Enzymes are proteins with a precise three-dimensional shape, especially at the active site, where the substrate binds. Changes in environmental conditions like temperature and pH can affect this structure either enhancing or disrupting enzyme function.
1. Temperature
Effect of Increasing Temperature
As temperature increases, enzyme activity also increases (up to a point). This is because molecules move faster, so more collisions occur between enzymes and substrates. The rate of reaction increases as a result.
Optimum Temperature
Each enzyme works best at a specific optimum temperature (for human enzymes, usually around 37°C—body temperature).
What Happens Above the Optimum?
At high temperatures, enzymes begin to denature. The active site loses its shape, so the substrate can no longer bind. Denaturation is irreversible. As a result, enzyme activity falls sharply after the optimum.
2. pH (Acidity/Alkalinity)
Effect of pH Changes
Just like temperature, enzymes have an optimum pH where they work best. This varies depending on the enzyme:
- Pepsin (in the stomach) works best at acidic pH ~2.
- Amylase (in the mouth) works best at neutral pH ~7.
- Trypsin (in the small intestine) works best at slightly alkaline pH ~8.
Too Acidic or Too Alkaline?
A pH that is too low or too high alters the enzyme’s structure, especially the active site. This reduces activity or may even cause denaturation. The enzyme can no longer bind the substrate properly.
Enzyme Activity vs. Temperature and pH – At a Glance
| Factor | What Helps? | What Harms? |
|---|---|---|
| Temperature | Moderate warmth (up to optimum, ~37°C) | High heat causes denaturation |
| pH | Enzyme’s specific optimum pH | Too acidic or too alkaline damages shape |
Tip to Remember
Bell-Shaped Curves
Both temperature and pH show a bell-shaped curve when graphed against enzyme activity. Activity rises to a peak (optimum) and then drops when conditions go beyond the ideal.
Summary
- Enzymes work best under optimal conditions.
- High temperatures and extreme pH levels can denature enzymes.
- Maintaining correct pH and temperature is essential for proper metabolism and digestion.
Lock-and-Key vs Induced Fit Models
Understanding How Enzymes Work
Enzymes are biological catalysts that speed up reactions by binding to specific molecules called substrates. The way an enzyme interacts with its substrate has been explained using two models: the Lock-and-Key model and the Induced Fit model.
1. Lock-and-Key Model
Concept
Proposed by Emil Fischer in 1894. It suggests that the active site of the enzyme has a specific shape that exactly matches the substrate like a key fitting into a lock.
Key Features
- The enzyme is rigid and does not change shape.
- Only substrates with the exact matching shape fit into the active site.
Analogy
Just like one specific key fits into one specific lock, only the correct substrate fits into the enzyme.
Limitation
This model does not explain how enzymes sometimes bind to similar but not identical substrates, or why enzymes slightly change shape during binding.
2. Induced Fit Model
Concept
Proposed by Daniel Koshland in 1958. It suggests that the enzyme’s active site is flexible and molds around the substrate during binding.
Key Features
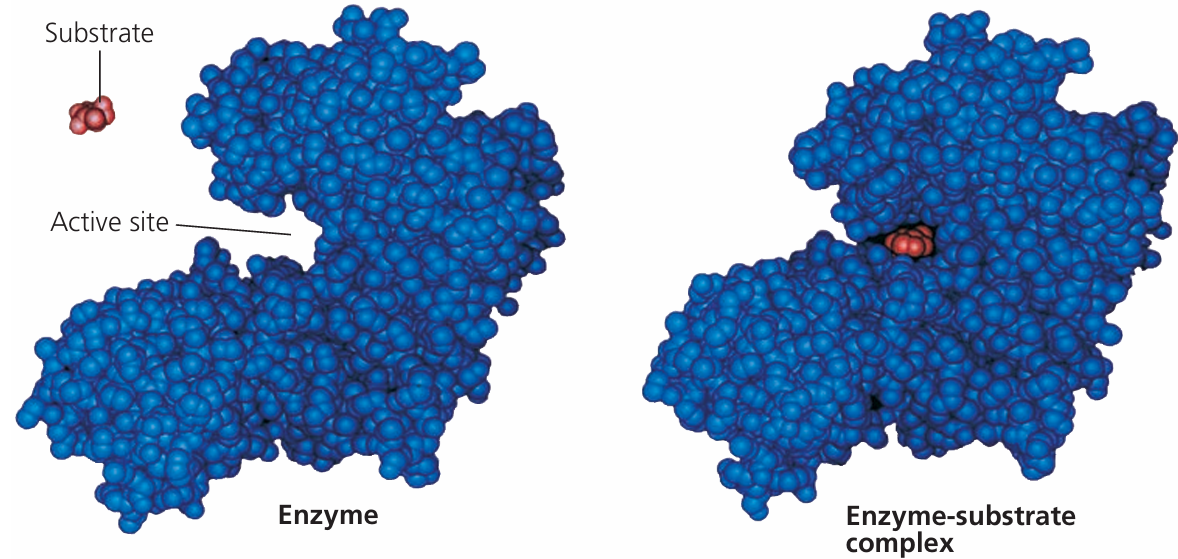
- Enzyme changes shape slightly to accommodate the substrate.
- The fit improves upon binding, which increases the efficiency of the reaction.
- Explains the flexibility of enzymes in recognizing substrates.
Analogy
Like a glove molding around a hand the glove adjusts to the hand’s shape for a perfect fit.
Comparison Table
| Feature | Lock-and-Key Model | Induced Fit Model |
|---|---|---|
| Enzyme Shape | Rigid | Flexible |
| Fit with Substrate | Exact fit only | Active site changes for better fit |
| Enzyme Specificity | Very high | High, with flexibility |
| Scientific Support | Early model | Widely accepted |
Why Induced Fit Is More Accepted Today
The Induced Fit model is supported by modern scientific evidence. It explains:
- How enzymes bind to substrates with similar shapes.
- The conformational changes that occur during binding.
- Why most enzymes are not completely rigid but adjust during the interaction.
Conclusion
- Both models help explain enzyme-substrate interaction and specificity.
- The Lock-and-Key model is simpler and easier to imagine.
- The Induced Fit model is more realistic and better supported by modern research.
