IB MYP 4-5 Biology-Osmosis- Study Notes - New Syllabus
IB MYP 4-5 Biology-Osmosis- Study Notes – New syllabus
IB MYP 4-5 Biology-Osmosis- Study Notes – IB MYP 4-5 Biology – per latest IB MYP Biology Syllabus.
Key Concepts:
- Water potential
- Hypertonic, hypotonic and isotonic solutions
- Osmosis in plant and animal cells
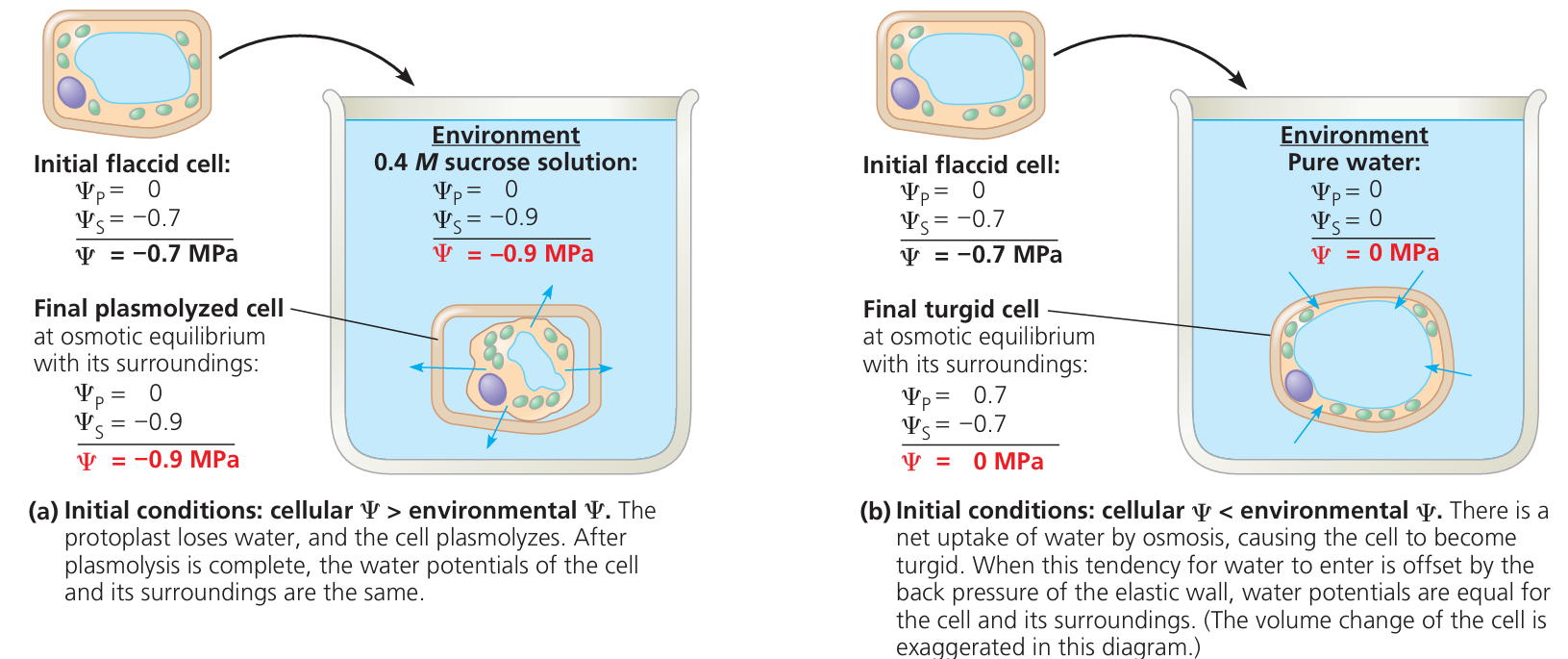
Water Potential
Osmosis is a type of diffusion, but specifically for water molecules.
It is the movement of water from a region of higher water potential to lower water potential.
It happens across a partially permeable membrane (a membrane that only allows some substances to pass through).
Definition of Osmosis
Osmosis is the diffusion of water molecules from a region of high-water potential to low water potential, through a partially permeable membrane.
What Is Water Potential?
- Water potential is a measure of the tendency of water to move from one place to another.
- Pure water has the highest water potential (usually given a value of zero).
- When substances (like salts or sugars) are dissolved in water, the water potential becomes lower (more negative).
Three Types of Osmosis Situations
| Solution Type | Description | Effect on Animal Cell | Effect on Plant Cell |
|---|---|---|---|
| Hypotonic | More water outside cell | Cell swells, may burst (lysis) | Cell becomes turgid (firm) |
| Isotonic | Same water potential inside and outside | No net movement | No change |
| Hypertonic | Less water outside cell | Cell shrinks (crenation) | Cell becomes plasmolysed |
Why Osmosis Matters in Cells
- Animal cells don’t have a cell wall. So, if too much water enters, they can burst.
- Plant cells have a strong cell wall. This gives them shape and prevents bursting. Instead, they become turgid or flaccid.
Turgor Pressure in Plant Cells
In a hypotonic solution, water enters the plant cell. The vacuole swells and presses against the cell wall. This pressure is called turgor pressure, and it helps keep plants upright and firm.
Plasmolysis in Plant Cells
In a hypertonic solution, water leaves the plant cell. The cytoplasm shrinks and pulls away from the cell wall. This condition is called plasmolysis, and the plant wilts.
Hypertonic, Hypotonic, and Isotonic Solutions
What Do These Terms Mean?
These three terms describe the concentration of solutes in a solution compared to the inside of a cell. They help us understand how water moves across the cell membrane during osmosis.
1. Hypotonic Solution
This means the water concentration is higher outside the cell.
Water moves into the cell.
- Animal cells swell and may burst (lysis).
- Plant cells become turgid (full and firm), which is ideal for plants.
Tip: Think “Hypo = Hippo” – the cell gets fat with water.
2. Isotonic Solution
Water moves in and out of the cell at equal rates.
There is no net gain or loss of water.
- Animal cells remain normal in shape – ideal for them.
- Plant cells become slightly soft (flaccid) but not damaged.
Tip: “Iso” means equal – water movement is balanced.
3. Hypertonic Solution
This means the water concentration is lower outside the cell.
Water moves out of the cell.
- Animal cells shrink or shrivel (crenation).
- Plant cells lose water, and their membrane pulls away from the wall (plasmolysis).
Tip: “Hyper” = more solute outside = water rushes out = cell shrinks.
Comparison Table
| Solution Type | Solute Concentration (Outside) | Water Movement | Effect on Animal Cell | Effect on Plant Cell |
|---|---|---|---|---|
| Hypotonic | Low | Into the cell | Cell swells or bursts | Cell becomes turgid |
| Isotonic | Equal | No net movement | Cell stays the same | Cell is flaccid |
| Hypertonic | High | Out of the cell | Cell shrinks (crenates) | Cell is plasmolysed (wilts) |
Why This Is Important
Understanding these solutions helps us explain:
- How cells maintain water balance
- Why IV fluids must be isotonic
- How plants stay firm (turgid) or wilt (plasmolysis)
Osmosis in Plant and Animal Cells
What Is Osmosis?
- Osmosis is the diffusion of water molecules across a partially permeable membrane from a region of higher water potential to a region of lower water potential.
- It’s how cells absorb or lose water – and how this affects their structure and function.
Osmosis in Animal Cells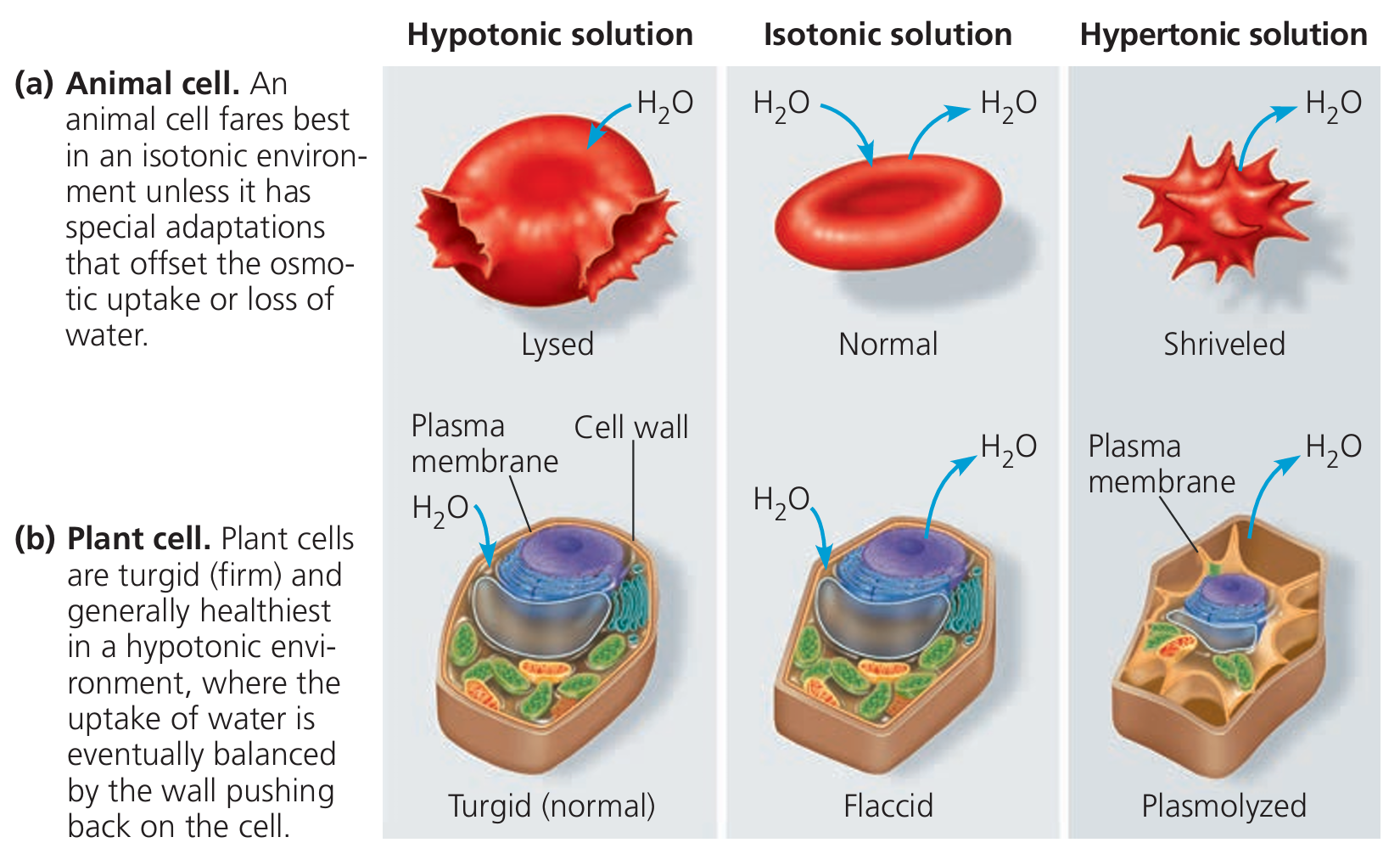
Animal cells (like red blood cells) lack a cell wall, so they are highly sensitive to osmosis.
Let’s see what happens in different solutions:
1. Hypotonic Solution
- Water enters the cell by osmosis.
- The cell swells and may burst (called lysis).
- No cell wall to stop it.
2. Isotonic Solution
- Water enters and leaves at the same rate.
- The cell stays normal in shape and size.
- Ideal for animal cells.
3. Hypertonic Solution
- Water moves out of the cell.
- The cell shrinks and becomes crenated.
- This can affect cell function.
Osmosis in Plant Cells
Plant cells have a rigid cell wall, which changes how they respond to osmosis.
1. Hypotonic Solution
- Water enters the cell.
- The vacuole swells and presses the cytoplasm against the cell wall.
- The cell becomes turgid (firm) – which is ideal for plant support.
2. Isotonic Solution
- Water enters and exits equally.
- The cell becomes slightly soft or flaccid.
- Plant wilts slightly.
3. Hypertonic Solution
- Water leaves the cell.
- The cell membrane pulls away from the cell wall.
- The cell becomes plasmolysed – the plant may wilt badly.
Summary: Effects of Osmosis
| Solution Type | Animal Cell | Plant Cell |
|---|---|---|
| Hypotonic | Swells, may burst | Turgid (best condition) |
| Isotonic | Normal shape | Flaccid (slightly soft) |
| Hypertonic | Shrinks (crenation) | Plasmolysis (wilting) |
Why Is This Important?
Understanding osmosis is essential for:
- How cells maintain water balance
- IV fluid composition in medicine
- Food preservation methods
- Plant structure and wilting mechanisms
