IB MYP 4-5 Biology-Word and Chemical Equations- Study Notes - New Syllabus
IB MYP 4-5 Biology-Word and Chemical Equations- Study Notes – New syllabus
IB MYP 4-5 Biology-Word and Chemical Equations- Study Notes – IB MYP 4-5 Biology – per latest IB MYP Biology Syllabus.
Key Concepts:
- Balancing chemical equations
- Photosynthesis: 6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
- Aerobic respiration: C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + energy
Word and Chemical Equations
Balancing is based on the Law of Conservation of Mass, which states that mass cannot be created or destroyed in a chemical reaction.
1. Word Equations
These describe a chemical reaction using names of substances.
Example (Aerobic respiration):
\[
\text{Glucose + Oxygen} \rightarrow \text{Carbon dioxide + Water + Energy (ATP)}
\]
This gives an idea of what reacts and what is formed, but not the exact quantities.
2. Chemical Equations
These use chemical formulas instead of names.
Unbalanced equation (for aerobic respiration):
\[
\mathrm{C_6H_{12}O_6 + O_2 \rightarrow CO_2 + H_2O}
\]
This is not yet balanced because the number of atoms of each element is not equal on both sides.
3. Balanced Chemical Equation
\mathrm{C_6H_{12}O_6 + 6O_2 \rightarrow 6CO_2 + 6H_2O}
\]
- Carbon: 6 atoms on both sides
- Hydrogen: 12 atoms on both sides
- Oxygen: 18 atoms on both sides
Steps to Balance Chemical Equations
- Write the unbalanced equation using correct formulas
- Count atoms of each element on both sides
- Use coefficients (numbers in front of formulas) to balance each element
- Start with elements that appear in only one compound on each side
- Balance hydrogen and oxygen last if they appear in multiple compounds
- Check your work to ensure the number of atoms is equal on both sides
- Note: Never change subscripts in a chemical formula, only coefficients
Examples:
1. Photosynthesis:
Word Equation:
Carbon dioxide + Water → Glucose + Oxygen
Balanced Chemical Equation:
\[
\mathrm{6CO_2 + 6H_2O \rightarrow C_6H_{12}O_6 + 6O_2}
\]
2. Anaerobic Respiration in Muscles:
Word Equation:
Glucose → Lactic acid + Energy (ATP)
Balanced Chemical Equation:
\[
\mathrm{C_6H_{12}O_6 \rightarrow 2C_3H_6O_3}
\]
Why Is Balancing Important in Biology?
- Shows exact amounts of reactants and products
- Reflects molecular conservation in biochemical processes
- Helps in stoichiometric calculations in lab experiments
- Makes biological reactions quantifiable and predictable
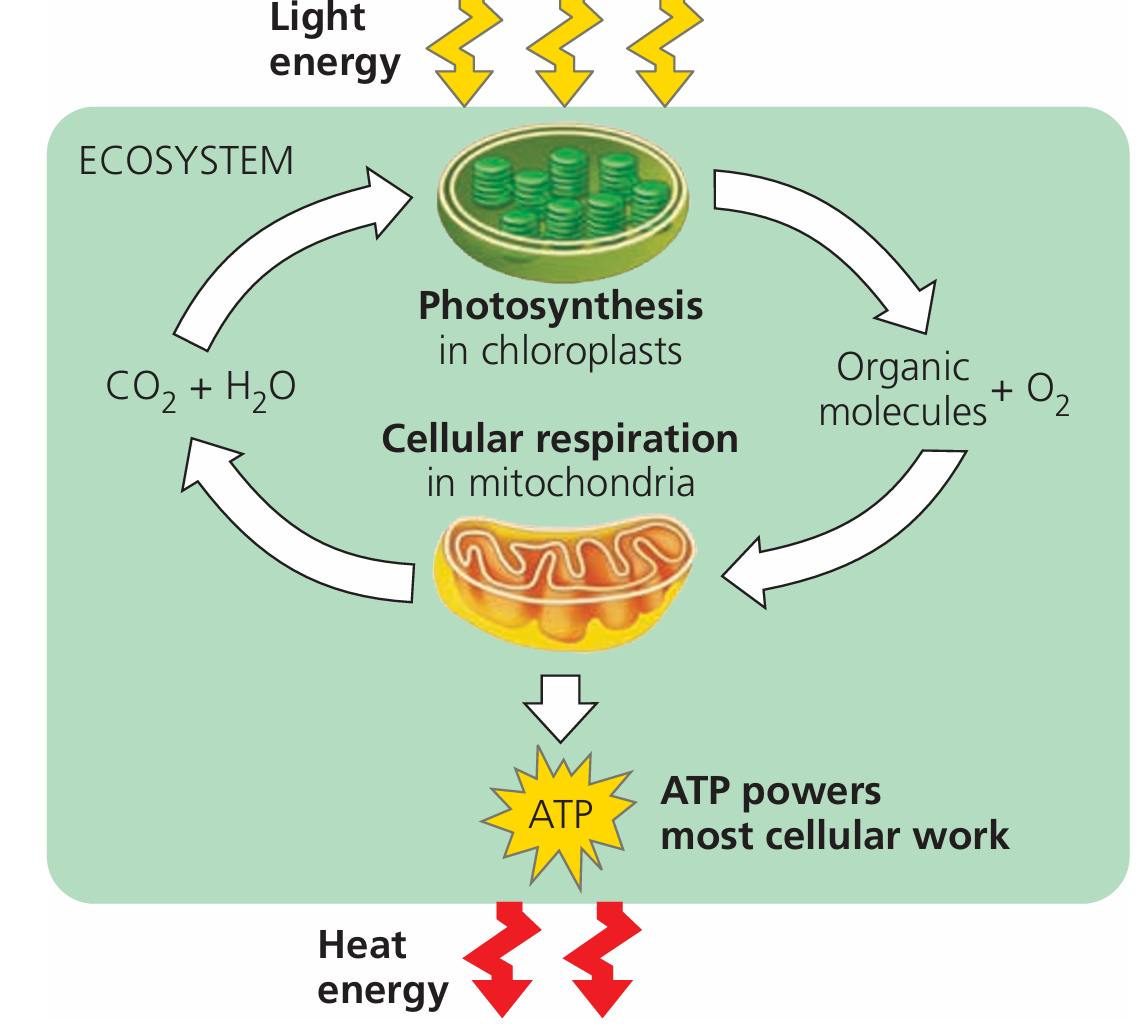
Photosynthesis
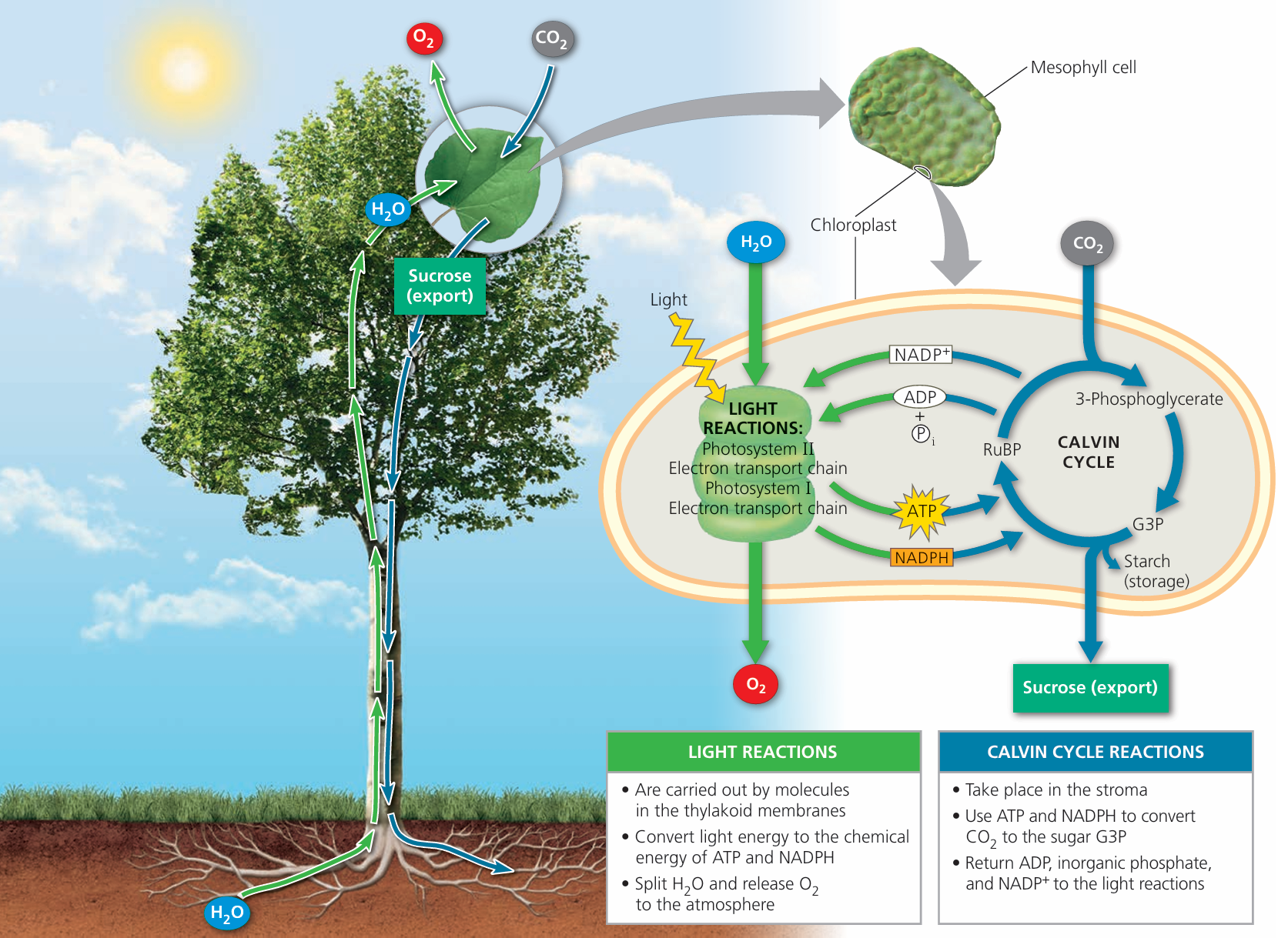
Introduction to Photosynthesis
Photosynthesis is a biochemical process in which green plants, algae, and some bacteria use sunlight to synthesize glucose from carbon dioxide and water.
This process occurs mainly in the chloroplasts of plant cells, using the green pigment chlorophyll to capture light energy.
Word Equation
(Using light energy and chlorophyll)
Balanced Chemical Equation
6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
- CO₂: Carbon dioxide (from the atmosphere)
- H₂O: Water (absorbed by roots)
- C₆H₁₂O₆: Glucose (stored chemical energy)
O₂: Oxygen (released as a by-product)
Breakdown of the Equation
| Reactants | Products |
|---|---|
| 6 molecules of CO₂ | 1 molecule of glucose (C₆H₁₂O₆) |
| 6 molecules of H₂O | 6 molecules of O₂ |
| Sunlight + chlorophyll | (Required for reaction to proceed) |
Key Points to Remember
- The equation is balanced and respects the conservation of carbon, hydrogen, and oxygen atoms.
- Glucose is the energy-storage molecule used later in respiration.
- Oxygen is released into the atmosphere and supports aerobic life.
Where Does It Occur?
- Location: In the chloroplasts of plant cells
- Pigment involved: Chlorophyll (absorbs blue and red light)
- Phase: Happens during daylight when sunlight is available
Biological Importance
- Provides the primary energy source for ecosystems
- Maintains atmospheric balance of oxygen and carbon dioxide
- Transforms inorganic substances into energy-rich organic molecules
Summary:
Photosynthesis is a vital biochemical reaction that traps light energy and stores it in glucose. It lays the foundation of the food chain and sustains nearly all life forms on Earth.
Aerobic Respiration
Introduction to Aerobic Respiration
Aerobic respiration is a biochemical process in which cells break down glucose in the presence of oxygen to release energy. This energy is stored as ATP (adenosine triphosphate), which powers cellular functions.
It takes place in the mitochondria of both plant and animal cells.
Word Equation
\text{Glucose + Oxygen} \rightarrow \text{Carbon dioxide + Water + Energy (ATP)}
\]
Balanced Chemical Equation
\[
\mathrm{C_6H_{12}O_6 + 6O_2 \rightarrow 6CO_2 + 6H_2O + ATP\ (Energy)}
\]
- C₆H₁₂O₆: Glucose
- O₂: Oxygen
- CO₂: Carbon dioxide
- H₂O: Water
- ATP: Usable form of energy
Why Is the Equation Balanced?
| Element | Reactants Count | Products Count |
|---|---|---|
| Carbon (C) | 6 (from glucose) | 6 (in CO₂) |
| Hydrogen (H) | 12 (from glucose) | 12 (in H₂O) |
| Oxygen (O) | 18 (6 from glucose + 6×2 from O₂) | 18 (12 in CO₂ + 6 in H₂O) |
Where Does It Occur?
- Location: Mitochondria of eukaryotic cells
- Conditions: Requires oxygen
- Organisms: Plants, animals, fungi, and protists
Biological Importance
- Generates a large amount of ATP (36–38 per glucose)
- Supports high-energy processes like:
- Muscle contraction
- Nerve signal transmission
- Active transport across membranes
- Cell division and repair
- Maintains homeostasis in complex organisms
Comparison: Aerobic vs. Anaerobic Respiration
| Feature | Aerobic Respiration | Anaerobic Respiration |
|---|---|---|
| Oxygen Required? | Yes | No |
| Location | Mitochondria | Cytoplasm |
| Products | CO₂, H₂O, ATP | Lactic acid or ethanol + CO₂ |
| ATP Yield | High (36–38 ATP) | Low (2 ATP) |
| End Use | Long-term energy | Short bursts of energy |