IB MYP Integrated Sciences e-Assessment : States and properties of matter
Exam Style Practice Questions - New Syllabus
Question (2 marks)
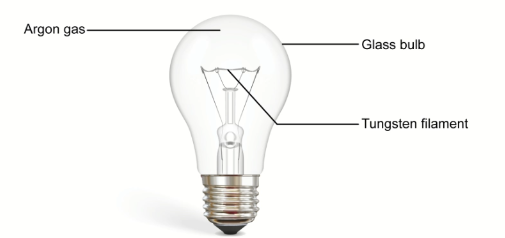
The diagram below shows an incandescent bulb. Incandescent light bulbs work when electricity passes through the filament and heats it. The filament is made out of tungsten (chemical symbol W) and is surrounded by argon (Ar) gas.
Describe two differences in the physical properties of tungsten and argon.
Tungsten property 1
Argon property 1
Tungsten property 2
Argon property 2
▶️Answer/Explanation
Ans:
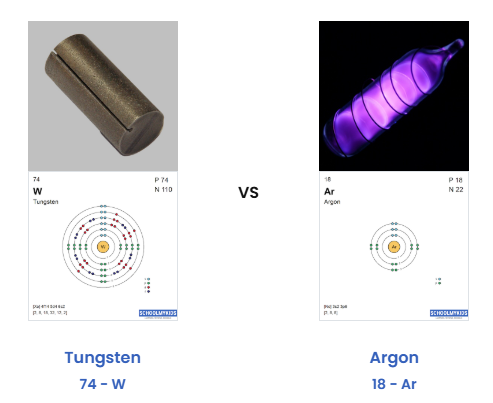
Tungsten Property 1: Tungsten is a solid at room temperature.
Argon Property 1: Argon is a gas at room temperature.
Tungsten Property 2:Tungsten has an extremely high melting point (3422 °C) and extremely high boiling point (5555 °C). This makes it possible for it to be exposed to the extreme heat in a light bulb.
Argon Property 2:Argon has a very low melting point (-189.3 °C) and very low boiling point (-185.9 °C). Argon is a gas even at very low temperatures.
