IB MYP 4-5 Chemistry -Acid + base, metal, or carbonate reactions- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Acid + base, metal, or carbonate reactions- Study Notes
Key Concepts
- Reactions of Acids with Bases, Metals, and Carbonates
Reactions of Acids with Bases, Metals, and Carbonates
Reactions of Acids with Bases, Metals, and Carbonates
Acids react with different classes of substances — bases, metals, and carbonates — in characteristic ways. These reactions help identify acids and are used widely in laboratory and industrial chemistry.
Reaction of Acid with Base — Neutralization
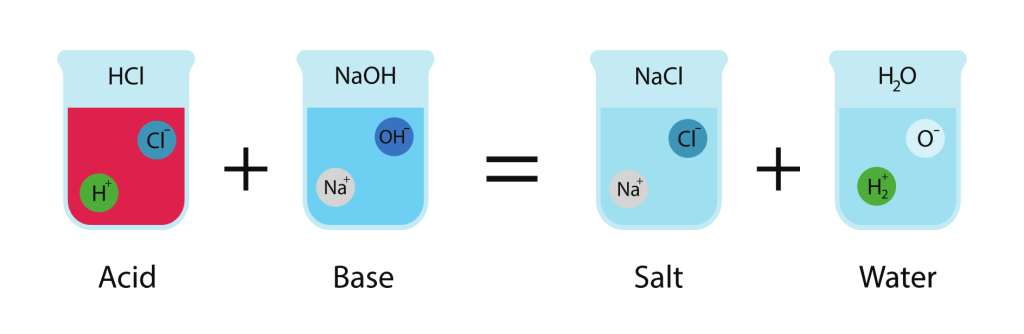
General Equation:
\( \mathrm{Acid + Base \rightarrow Salt + Water} \)
Ionic Equation:
\( \mathrm{H^+(aq) + OH^-(aq) \rightarrow H_2O(l)} \)
Explanation:
- Acids provide hydrogen ions \( \mathrm{(H^+)} \), bases provide hydroxide ions \( \mathrm{(OH^-)} \).
- These ions combine to form water, while the remaining ions form a salt.
Example: \( \mathrm{HCl + NaOH \rightarrow NaCl + H_2O} \)
Reaction of Acid with Metal
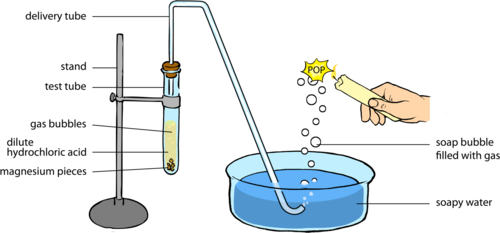
General Equation:
\( \mathrm{Acid + Metal \rightarrow Salt + Hydrogen\ gas} \)
Explanation:
- Metals above hydrogen in the reactivity series react with acids.
- Hydrogen gas is released during the reaction.
- Salt formed depends on the acid and the metal used.
Examples:
- \( \mathrm{Mg + 2HCl \rightarrow MgCl_2 + H_2} \)
- \( \mathrm{Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2} \)
Observation: Effervescence (bubbling) occurs due to hydrogen gas production.
Reaction of Acid with Carbonate (or Hydrogen Carbonate)
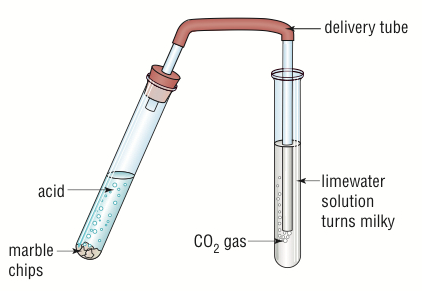
General Equation:
\( \mathrm{Acid + Carbonate \rightarrow Salt + Water + Carbon\ dioxide} \)
or
\( \mathrm{Acid + Hydrogen\ carbonate \rightarrow Salt + Water + Carbon\ dioxide} \)
Explanation:
- Acid reacts with a carbonate or bicarbonate to produce carbon dioxide gas, which causes effervescence.
- The reaction is used as a test for carbonates — gas turns limewater milky.
Examples:
- \( \mathrm{CaCO_3 + 2HCl \rightarrow CaCl_2 + H_2O + CO_2} \)
- \( \mathrm{NaHCO_3 + HCl \rightarrow NaCl + H_2O + CO_2} \)
Acid Reactions
| Type of Substance Reacting with Acid | General Reaction | Products Formed | Example Equation |
|---|---|---|---|
| Base (Alkali) | Acid + Base → Salt + Water | Neutralization product | \( \mathrm{HCl + NaOH \rightarrow NaCl + H_2O} \) |
| Metal | Acid + Metal → Salt + Hydrogen gas | Salt + \( \mathrm{H_2} \) | \( \mathrm{Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2} \) |
| Carbonate | Acid + Carbonate → Salt + Water + CO₂ | Salt + \( \mathrm{CO_2} + \mathrm{H_2O} \) | \( \mathrm{CaCO_3 + 2HCl \rightarrow CaCl_2 + H_2O + CO_2} \) |
Laboratory Observations for Each Reaction
| Reaction Type | Observable Effect | Gas Produced | Gas Test |
|---|---|---|---|
| Acid + Base | Temperature rises slightly | None | – |
| Acid + Metal | Effervescence / bubbling | Hydrogen | Pops with a lighted splint |
| Acid + Carbonate | Fizzing / gas bubbles | Carbon dioxide | Turns limewater milky |
Example
Write a balanced equation for the reaction between hydrochloric acid and magnesium.
▶️ Answer / Explanation
Step 1: Magnesium reacts with acid to produce hydrogen gas and salt.
Step 2: \( \mathrm{Mg + 2HCl \rightarrow MgCl_2 + H_2} \)
Final Answer: Magnesium chloride and hydrogen gas are formed, with visible effervescence.
Example
Describe the reaction between sulfuric acid and sodium carbonate. What test confirms the gas produced?
▶️ Answer / Explanation
Step 1: \( \mathrm{Na_2CO_3 + H_2SO_4 \rightarrow Na_2SO_4 + H_2O + CO_2} \)
Step 2: Effervescence due to carbon dioxide gas.
Step 3: Passing gas through limewater turns it milky — confirms \( \mathrm{CO_2} \).
Final Answer: Sodium sulfate, water, and carbon dioxide are formed.
Example
A student mixes nitric acid with calcium hydroxide and collects the resulting solution. Identify the salt formed and explain how you could confirm neutralization.
▶️ Answer / Explanation
Step 1: Reaction: \( \mathrm{2HNO_3 + Ca(OH)_2 \rightarrow Ca(NO_3)_2 + 2H_2O} \)
Step 2: Salt formed is calcium nitrate.
Step 3: Neutralization can be confirmed using litmus — final solution is nearly neutral (pH ≈ 7).
Final Answer: Calcium nitrate is formed; no effervescence; temperature rise indicates exothermic neutralization.
