IB MYP 4-5 Chemistry -Alcohols- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Alcohols- Study Notes
Key Concepts
- Alcohols (Structure, Properties, and Reactions)
Alcohols (Structure, Properties, and Reactions)
Alcohols (Structure, Properties, and Reactions)
Alcohols are organic compounds that contain one or more hydroxyl groups (–OH) attached to a carbon atom. They belong to a homologous series with the general formula \( \mathrm{C_{n}H_{2n+1}OH} \).
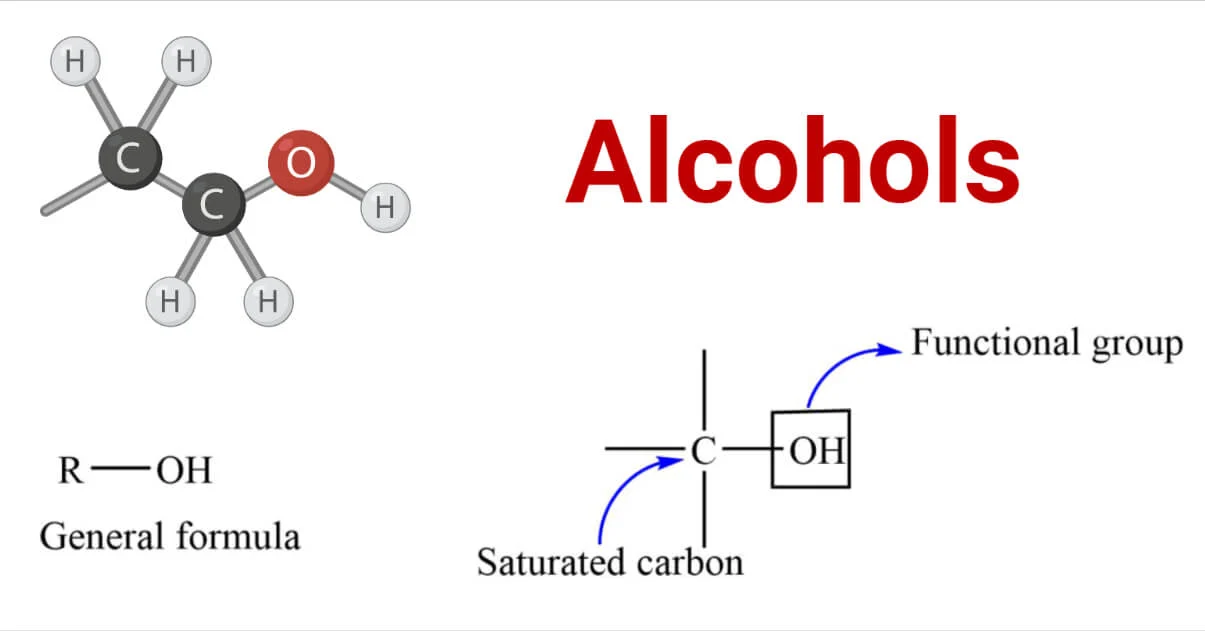
General Formula and Classification
- General formula: \( \mathrm{C_{n}H_{2n+1}OH} \)
- Functional group: Hydroxyl group (–OH)
- Homologous series: Alcohols
Types of Alcohols (based on –OH position):
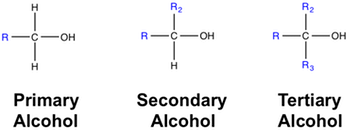
- Primary (1°) alcohol: –OH attached to a carbon bonded to only one other carbon atom. Example: Ethanol (\( \mathrm{CH_3CH_2OH} \))
- Secondary (2°) alcohol: –OH attached to a carbon bonded to two other carbons. Example: Propan-2-ol (\( \mathrm{CH_3CHOHCH_3} \))
- Tertiary (3°) alcohol: –OH attached to a carbon bonded to three other carbons. Example: 2-Methylpropan-2-ol (\( \mathrm{(CH_3)_3COH} \))
Structural Formula and Naming
| Name | Molecular Formula | Structural Formula |
|---|---|---|
| Methanol | \( \mathrm{CH_3OH} \) | H–CH₂–OH |
| Ethanol | \( \mathrm{C_2H_5OH} \) | CH₃–CH₂–OH |
| Propan-1-ol | \( \mathrm{C_3H_7OH} \) | CH₃–CH₂–CH₂–OH |
Preparation of Alcohols![]()
- (a) Fermentation of sugars: Glucose is converted into ethanol and carbon dioxide using yeast.
\( \mathrm{C_6H_{12}O_6 \xrightarrow{yeast} 2C_2H_5OH + 2CO_2} \)
- (b) Hydration of alkenes: Ethene reacts with steam in the presence of phosphoric acid catalyst to form ethanol.
\( \mathrm{C_2H_4 + H_2O \xrightarrow{H_3PO_4} C_2H_5OH} \)
Physical Properties
- Colorless liquids at room temperature (lower alcohols).
- Boiling point increases with molecular mass (due to hydrogen bonding).
- Soluble in water — solubility decreases with chain length.
- Pleasant smell and flammable.
Chemical Reactions of Alcohols
(a) Combustion: Alcohols burn in air to form carbon dioxide and water, releasing energy.
\( \mathrm{C_2H_5OH + 3O_2 \rightarrow 2CO_2 + 3H_2O + energy} \)
(b) Oxidation: Alcohols can be oxidized to aldehydes, carboxylic acids, or ketones using oxidizing agents (like \( \mathrm{K_2Cr_2O_7/H_2SO_4} \)).

\( \mathrm{CH_3CH_2OH \xrightarrow{[O]} CH_3CHO \xrightarrow{[O]} CH_3COOH} \)
(c) Dehydration (Elimination): When heated with concentrated \( \mathrm{H_2SO_4} \), ethanol loses water to form ethene.

\( \mathrm{C_2H_5OH \xrightarrow{conc.\ H_2SO_4} C_2H_4 + H_2O} \)
Uses of Alcohols![]()
- As fuels (ethanol in biofuels).
- As solvents in perfumes, medicines, and paints.
- As disinfectants and antiseptics.
- In industrial synthesis (to make esters, acids, etc.).
- Methanol is used as a feedstock in plastics and formaldehyde production.
Comparison: Alcohols vs. Hydrocarbons
| Property | Hydrocarbons | Alcohols |
|---|---|---|
| Functional Group | None | –OH (Hydroxyl) |
| Polarity | Nonpolar | Polar (forms hydrogen bonds) |
| Solubility in Water | Insoluble | Soluble (lower alcohols) |
| Reactivity | Low | High (undergo oxidation, dehydration) |
Example
Write the balanced chemical equation for the combustion of methanol.
▶️ Answer / Explanation
Step 1: Methanol = \( \mathrm{CH_3OH} \)
Step 2: Combustion produces carbon dioxide and water.
Step 3: \( \mathrm{2CH_3OH + 3O_2 \rightarrow 2CO_2 + 4H_2O} \)
Final Answer: Methanol burns in air to produce \( \mathrm{CO_2} \) and \( \mathrm{H_2O} \), releasing energy.
Example
Describe a chemical test to distinguish ethanol from ethene.
▶️ Answer / Explanation
Step 1: Add bromine water to both samples.
Step 2: Ethene (unsaturated) decolorizes bromine water.
Step 3: Ethanol (saturated) does not affect bromine water.
Final Answer: Bromine water turns colorless with ethene but remains orange with ethanol.
Example
Calculate the mass of carbon dioxide produced from complete combustion of 46 g of ethanol (\( \mathrm{C_2H_5OH} \)).
▶️ Answer / Explanation
Step 1: Balanced equation: \( \mathrm{C_2H_5OH + 3O_2 \rightarrow 2CO_2 + 3H_2O} \)
Step 2: Molar mass of ethanol = 46 g/mol → produces 2 mol \( \mathrm{CO_2} \).
Step 3: Molar mass of \( \mathrm{CO_2} = 44\ g/mol \)
Step 4: Mass of \( \mathrm{CO_2} = 2 \times 44 = 88\ g \)
Final Answer: 46 g of ethanol produces 88 g of \( \mathrm{CO_2} \) on complete combustion.
