IB MYP 4-5 Chemistry -Alpha, beta, and gamma radiation- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Alpha, beta, and gamma radiation- Study Notes
Key Concepts
- Alpha, Beta, and Gamma Radiation
Alpha, Beta, and Gamma Radiation
Alpha, Beta, and Gamma Radiation
When unstable atomic nuclei decay, they emit energy and particles in the form of nuclear radiation. The three main types of radiation are alpha (α), beta (β), and gamma (γ) radiation. Each type has distinct properties and effects based on its mass, charge, and penetrating power.
Alpha, beta, and gamma radiations are forms of ionizing radiation — they can remove electrons from atoms and molecules, forming ions.
Alpha (α) Radiation
Nature: An alpha particle is a helium nucleus consisting of 2 protons and 2 neutrons, represented as \( \mathrm{^4_2He^{2+}} \).![]()
Properties:
- Positively charged (+2).
- Relatively heavy (mass = 4 atomic units).
- Low penetration power — stopped by paper or skin.
- High ionizing power — causes dense ionization in air.
Example of Alpha Decay:
\( \mathrm{^{238}_{92}U \rightarrow ^{234}_{90}Th + ^4_2He} \)
Here, uranium-238 decays into thorium-234 by emitting an alpha particle.
Effect on the Nucleus:
- Mass number decreases by 4.
- Atomic number decreases by 2 (new element formed).
Beta (β⁻) Radiation
Nature: A beta particle is a high-speed electron emitted when a neutron in the nucleus changes into a proton and an electron.![]()
Properties:
- Negatively charged (−1).
- Very light (mass ≈ 1/1836 of a proton).
- Moderate penetration — passes through paper but stopped by thin aluminum sheet.
- Moderate ionizing power.
Example of Beta Decay:
\( \mathrm{^{14}_6C \rightarrow ^{14}_7N + ^0_{-1}e} \)
Carbon-14 decays into nitrogen-14 by emitting a beta particle.
Effect on the Nucleus:
- Mass number remains unchanged.
- Atomic number increases by 1 (one neutron becomes a proton).
Gamma (γ) Radiation
Nature: Gamma radiation is a high-energy electromagnetic wave emitted from the nucleus. It usually follows alpha or beta decay as the nucleus releases excess energy.
Properties: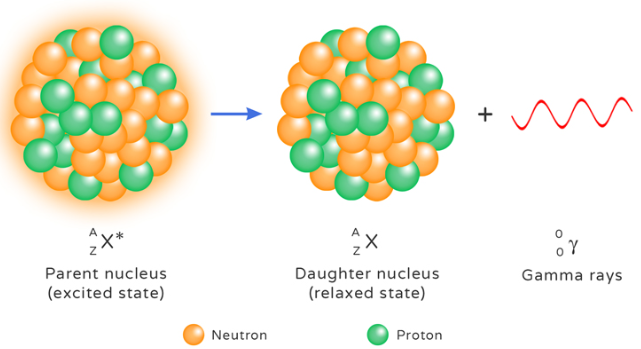
- No charge (neutral).
- No mass — it is pure energy (photon).
- Very high penetration — can pass through several centimeters of lead or meters of concrete.
- Weak ionizing power but highly penetrating.
Example of Gamma Emission:
\( \mathrm{^{60}_{27}Co^* \rightarrow ^{60}_{27}Co + \gamma} \)
Here, cobalt-60 emits gamma rays as it moves from an excited state to a stable state.
Effect on the Nucleus:
- No change in mass number or atomic number — only energy is lost.
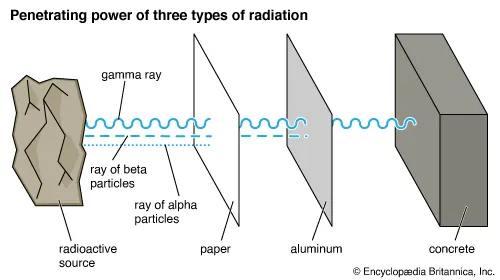
Comparative Properties of α, β, and γ Radiation
| Property | Alpha (α) | Beta (β⁻) | Gamma (γ) |
|---|---|---|---|
| Nature | Helium nucleus (2p, 2n) | High-speed electron | Electromagnetic wave |
| Charge | +2 | −1 | 0 |
| Mass | 4 u | 1/1836 u | 0 |
| Speed | Slow (1/10 of light) | Fast (close to light speed) | Speed of light |
| Penetrating Power | Low | Medium | Very High |
| Ionizing Power | High | Moderate | Low |
| Stopped By | Paper or skin | Thin aluminum sheet | Thick lead or concrete |
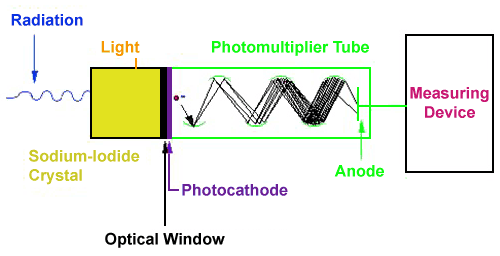
Detection of Radiation
- Geiger-Müller (GM) counter: Detects and measures ionizing radiation by counting pulses produced by gas ionization.
- Photographic film: Darkens when exposed to radiation; used in dosimeters for safety monitoring.
- Scintillation detectors: Detect flashes of light produced when radiation hits a special material.
Effects and Safety Precautions
- Effects of exposure: Damage to cells, DNA mutations, burns, cancer, or radiation sickness.
- Protection: Use lead shielding, minimize exposure time, and maintain safe distance from sources.
Summary of α, β, and γ Radiations
| Radiation Type | Change in Nucleus | Penetration | Effect on Element |
|---|---|---|---|
| Alpha (α) | Mass −4, Atomic no. −2 | Low | New element formed |
| Beta (β⁻) | Mass same, Atomic no. +1 | Medium | New element formed |
| Gamma (γ) | No change | Very high | Same element, lower energy state |
Example
Write the nuclear equation for the alpha decay of radium-226.
▶️ Answer / Explanation
Step 1: Alpha decay releases a helium nucleus \( \mathrm{^4_2He} \).
Step 2: \( \mathrm{^{226}_{88}Ra \rightarrow ^{222}_{86}Rn + ^4_2He} \)
Final Answer: Radium-226 decays to radon-222 by emitting an alpha particle.
Example
How does the emission of a beta particle change the atomic number and mass number of an atom?
▶️ Answer / Explanation
Step 1: In beta decay, a neutron converts into a proton and an electron.
Step 2: The proton stays in the nucleus; the electron (β⁻) is emitted.
Step 3: Atomic number increases by 1, but mass number remains unchanged.
Final Answer: Beta decay increases atomic number by 1 while keeping the mass number constant.
Example
Compare the ionizing and penetrating powers of alpha, beta, and gamma radiation, explaining the reason for the difference.
▶️ Answer / Explanation
Step 1: Alpha particles are massive and carry +2 charge, so they collide strongly with atoms — high ionizing power, low penetration.
Step 2: Beta particles are lighter and less charged, producing moderate ionization and medium penetration.
Step 3: Gamma rays have no mass or charge; they interact weakly — low ionization but very high penetration.
Final Answer: Ionizing power decreases while penetrating power increases from α → β → γ due to differences in mass and charge.
