IB MYP 4-5 Chemistry -Applications in medicine and industry- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Applications in medicine and industry- Study Notes
Key Concepts
- Applications of Radioactivity in Medicine and Industry
Applications of Radioactivity in Medicine and Industry
Applications of Radioactivity in Medicine and Industry
Radioactive isotopes (radioisotopes) are unstable atoms that emit radiation as they decay to become more stable.
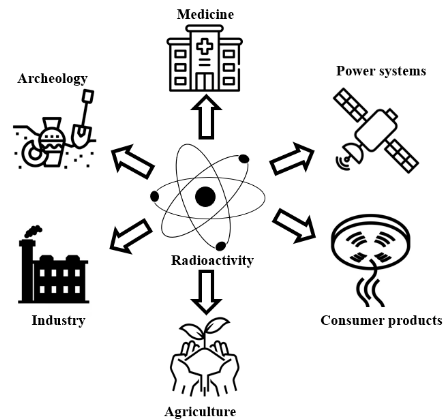
This property makes them extremely useful in medicine and industry for tracing, imaging, treatment, and quality control purposes.
Applications in Medicine
Radioisotopes are widely used in healthcare for both diagnosis (detecting diseases) and therapy (treating diseases).
A. Diagnostic Uses (Tracers and Imaging)
- Radioactive tracers help visualize and track processes inside the body without surgery.
- Small amounts of a radioactive isotope are injected or swallowed, and detectors (like gamma cameras) trace their movement.
- Common isotopes: \( \mathrm{^{99m}Tc} \) (Technetium-99m), \( \mathrm{^{131}I} \) (Iodine-131), and \( \mathrm{^{201}Tl} \) (Thallium-201).
Examples:
- \( \mathrm{^{99m}Tc} \) — used to scan the brain, lungs, and kidneys.
- \( \mathrm{^{131}I} \) — used for thyroid gland imaging and treatment.
- \( \mathrm{^{201}Tl} \) — used in heart function studies.
B. Therapeutic Uses (Treatment)
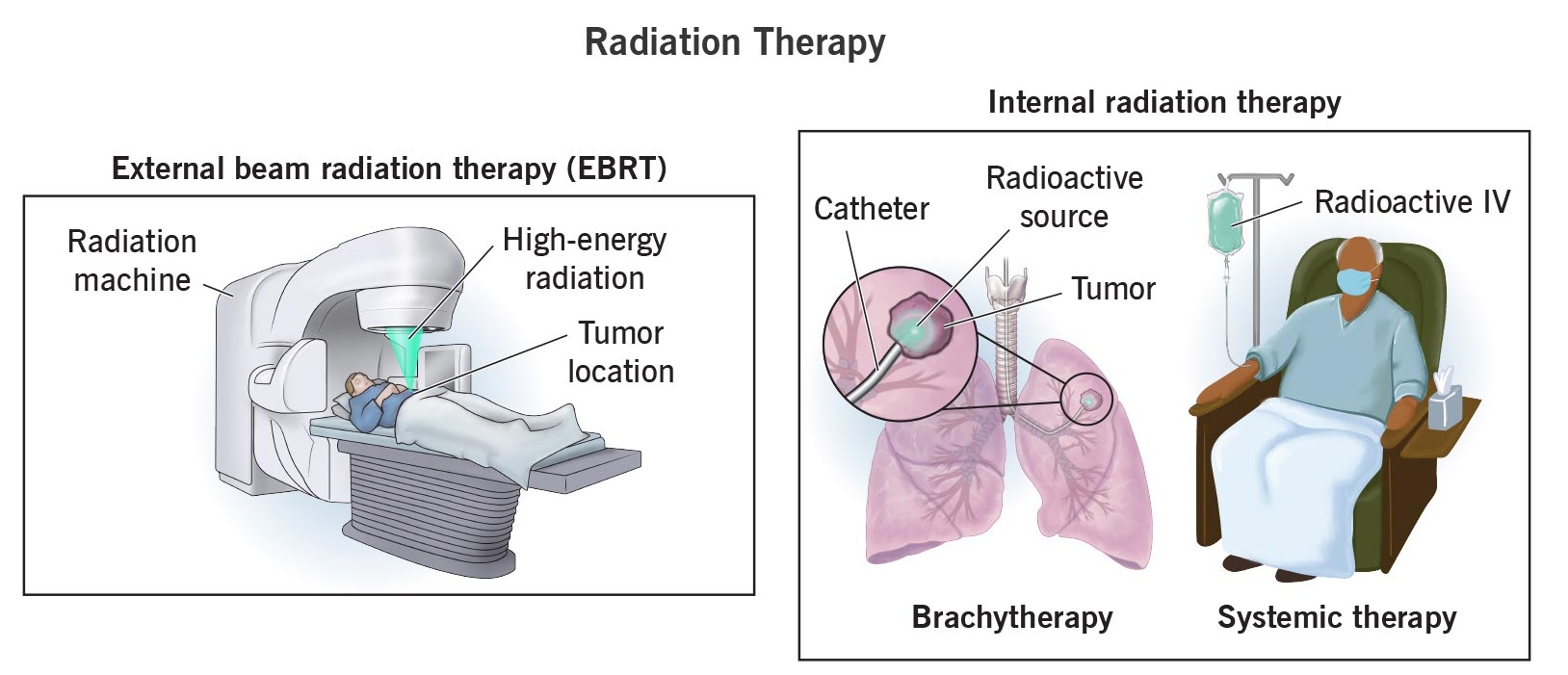
- Radiotherapy: High-energy gamma rays or beta radiation are used to kill cancer cells.
- \( \mathrm{^{60}Co} \) (Cobalt-60) and \( \mathrm{^{137}Cs} \) (Cesium-137) emit gamma rays that target and destroy tumors.
- Thyroid Treatment: \( \mathrm{^{131}I} \) is used to shrink or destroy overactive thyroid tissue.
C. Sterilization:
- Gamma rays sterilize medical tools, surgical gloves, and syringes without heat.
- Used for heat-sensitive equipment and food sterilization in hospitals.
Common Radioisotopes in Medicine
| Isotope | Use | Type of Radiation | Reason for Suitability |
|---|---|---|---|
| \( \mathrm{^{99m}Tc} \) | Imaging of organs (brain, kidneys, heart) | γ | Short half-life (6 h), low tissue damage |
| \( \mathrm{^{131}I} \) | Thyroid treatment and scans | β, γ | Accumulates in thyroid, effective treatment |
| \( \mathrm{^{60}Co} \) | Cancer radiotherapy | γ | Penetrating radiation kills deep tumor cells |
Applications in Industry
Radioisotopes play an important role in manufacturing, energy, and engineering by allowing non-destructive testing, tracing, and process control.
A. Thickness and Density Measurement
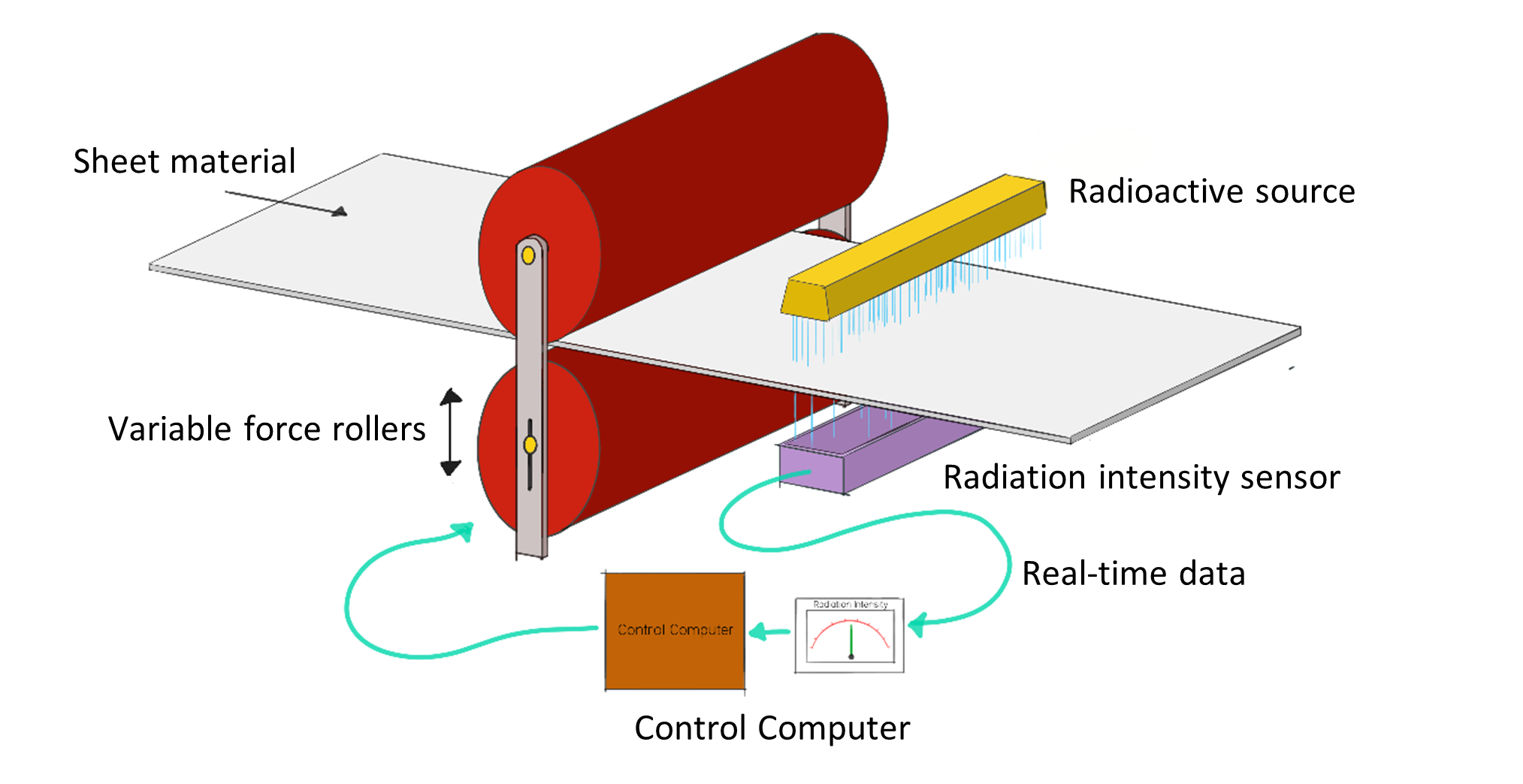
- Beta radiation is used to measure material thickness (e.g., paper, metal sheets, plastic films).
- The detector measures how much radiation passes through — less radiation means thicker material.
- Isotopes used: \( \mathrm{^{90}Sr} \), \( \mathrm{^{147}Pm} \).
B. Leak Detection and Flow Tracing

- Radioactive tracers detect leaks in pipelines and check the flow of liquids in closed systems.
- For example, \( \mathrm{^{24}Na} \) is added to detect leaks in underground water pipes.
C. Power Generation (Nuclear Reactors)

- \( \mathrm{^{235}U} \) (Uranium-235) and \( \mathrm{^{239}Pu} \) (Plutonium-239) are used as fuels in nuclear reactors.
- They undergo nuclear fission to produce large amounts of energy for electricity generation.
D. Material Sterilization and Preservation
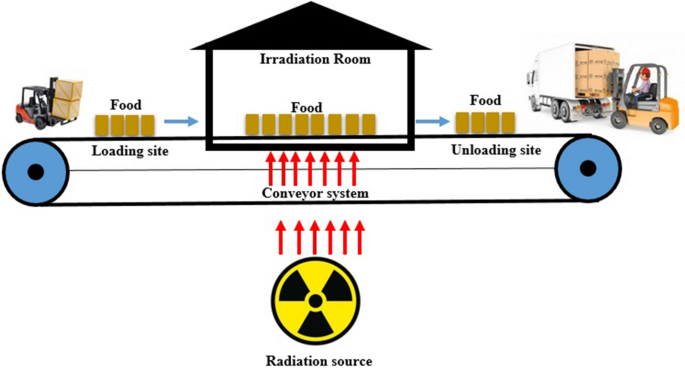
- Gamma radiation sterilizes food and industrial tools to prevent spoilage and microbial growth.
Common Radioisotopes in Industry
| Isotope | Use | Type of Radiation |
|---|---|---|
| \( \mathrm{^{90}Sr} \) | Thickness control in manufacturing | β |
| \( \mathrm{^{60}Co} \) | Sterilization of food and equipment | γ |
| \( \mathrm{^{24}Na} \) | Leak detection in pipelines | γ |
Safety Considerations

- Medical staff and workers must wear lead aprons and dosimeters to monitor exposure.
- Time, distance, and shielding principles minimize radiation risk.
- Radioisotopes used in medicine have short half-lives to reduce patient exposure.
- Industrial applications use automated systems to minimize human contact.
Example
Explain how \( \mathrm{^{131}I} \) is used to both diagnose and treat thyroid disorders.
▶️ Answer / Explanation
Step 1: The thyroid gland naturally absorbs iodine.
Step 2: \( \mathrm{^{131}I} \) emits beta and gamma radiation that helps visualize gland function and destroy overactive cells.
Final Answer: \( \mathrm{^{131}I} \) is effective because it accumulates in the thyroid and emits radiation useful for both imaging and therapy.
Example
Why is \( \mathrm{^{99m}Tc} \) preferred as a medical tracer compared to other isotopes?
▶️ Answer / Explanation
Step 1: It emits only gamma radiation — easily detected but causes minimal tissue damage.
Step 2: It has a short half-life (~6 hours), so radiation exposure is brief.
Step 3: It forms compounds that target specific organs.
Final Answer: \( \mathrm{^{99m}Tc} \) is ideal because it provides clear imaging with low radiation risk and rapid decay.
Example
Discuss how radioisotopes improve industrial safety and product quality while also posing potential hazards.
▶️ Answer / Explanation
Step 1: Radioisotopes ensure consistent product thickness and detect hidden leaks without damaging materials.
Step 2: This improves production accuracy and safety monitoring.
Step 3: However, mishandling or poor shielding can expose workers to harmful radiation.
Final Answer: Radioisotopes enhance quality control and safety but require strict handling procedures to prevent radiation hazards.
