IB MYP 4-5 Chemistry -Bond strength, melting points, and conductivity- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Bond strength, melting points, and conductivity- Study Notes
Key Concepts
- Bond strength, melting points, and conductivity
Bond strength, melting points, and conductivity
Bond Strength
Definition: Bond strength is a measure of how strongly atoms are held together within a compound. It is the energy required to break one mole of bonds in gaseous molecules called the bond dissociation energy.
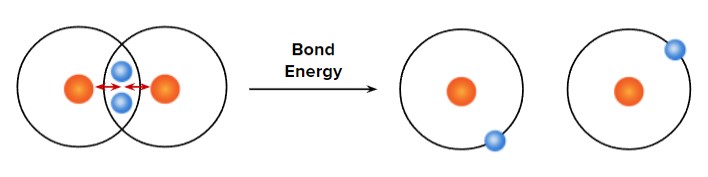
Bond strength ∝ \( \mathrm{\dfrac{1}{bond\ length}} \)
- Stronger bonds → higher bond energy → shorter bond length.
- Triple bonds are stronger than double, which are stronger than single bonds.
Order of Bond Strength: \( \mathrm{C \equiv C > C = C > C – C} \)
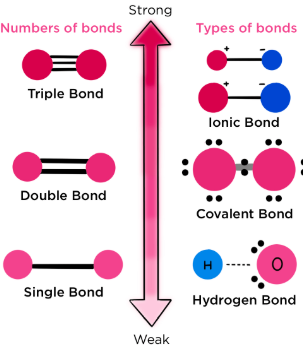
Factors Affecting Bond Strength:
- Bond type (ionic, covalent, metallic)
- Bond length — shorter bonds are stronger
- Electronegativity difference — greater difference → stronger ionic character
| Bond Type | Bond Strength (Relative) | Explanation |
|---|---|---|
| Ionic Bond (e.g., NaCl) | Very strong | Electrostatic attraction between positive and negative ions. |
| Covalent Bond (e.g., HCl) | Strong | Atoms share electrons to achieve stability. |
| Metallic Bond (e.g., Cu) | Variable strength | Attraction between metal cations and delocalized electrons. |
Example
Between \( \mathrm{H–H} \) and \( \mathrm{H–Cl} \) bonds, which is stronger and why?
▶️ Answer / Explanation
Step 1: Bond energy of \( \mathrm{H–H} \) = 436 kJ/mol; \( \mathrm{H–Cl} \) = 431 kJ/mol.
Step 2: The \( \mathrm{H–H} \) bond is slightly stronger due to shorter bond length and higher overlap efficiency.
Final Answer: \( \mathrm{H–H} \) bond is stronger than \( \mathrm{H–Cl} \) bond.
Example
Compare the bond strength of \( \mathrm{N \equiv N} \) and \( \mathrm{O = O} \).
▶️ Answer / Explanation
Step 1: \( \mathrm{N_2} \) has a triple bond; \( \mathrm{O_2} \) has a double bond.
Step 2: Triple bonds are stronger due to three shared electron pairs.
Final Answer: \( \mathrm{N \equiv N} \) is stronger than \( \mathrm{O = O} \).
Melting and Boiling Points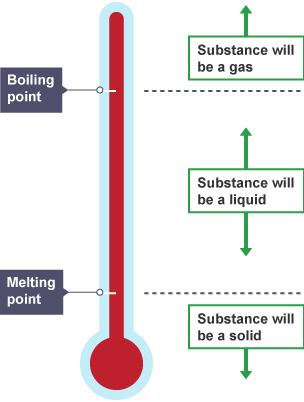
Definition: The melting point is the temperature at which a solid changes to a liquid. The boiling point is when a liquid changes to a gas.
These depend on the forces between particles — the stronger the forces, the higher the melting or boiling point.
Factors Affecting Melting and Boiling Points:
- Bond strength — stronger bonds → higher melting/boiling points.
- Type of bonding — ionic > metallic > covalent (molecular).
- Molecular size — larger molecules → stronger van der Waals forces.
- Structure — giant lattices have higher melting points than simple molecules.
| Substance Type | Bonding Type | Melting Point | Example |
|---|---|---|---|
| Ionic Compounds | Ionic bonds | High (e.g., 800–1000°C) | NaCl |
| Covalent Network | Strong covalent | Very high (e.g., >3000°C) | Diamond (C) |
| Molecular Compounds | Weak van der Waals | Low (<100°C) | I₂, CH₄ |
Example
Explain why NaCl has a much higher melting point than CH₄.
▶️ Answer / Explanation
Step 1: NaCl is ionic; CH₄ is covalent.
Step 2: Ionic bonds are much stronger than weak van der Waals forces in CH₄.
Final Answer: NaCl has a higher melting point due to strong electrostatic forces between ions.
Example
Why does graphite have a high melting point even though it conducts electricity?
▶️ Answer / Explanation
Step 1: Graphite has strong covalent bonds between carbon atoms in layers.
Step 2: A large amount of energy is needed to break these bonds.
Final Answer: Graphite has a high melting point due to its strong covalent network.
Electrical Conductivity
Definition: Electrical conductivity is the ability of a substance to allow electric current to pass through it. It depends on the presence of mobile charged particles (electrons or ions).
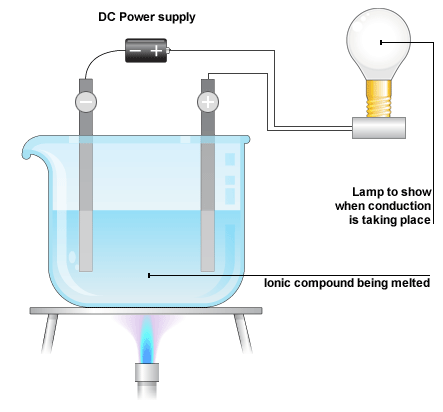
Key Points:
- Metals: Conduct electricity due to free-moving delocalized electrons.
- Ionic compounds: Conduct electricity only when molten or dissolved (ions are free to move).
- Covalent compounds: Do not conduct electricity because they have no free electrons or ions.
| Substance Type | Conductivity in Solid State | Conductivity in Molten/Aqueous State | Example |
|---|---|---|---|
| Metal | Good | Good | Copper (Cu) |
| Ionic Compound | Poor (ions fixed) | Good (ions mobile) | NaCl |
| Covalent Compound | Poor | Poor | CH₄, H₂O |
Example
Why does solid NaCl not conduct electricity, but molten NaCl does?
▶️ Answer / Explanation
Step 1: In solid NaCl, ions are fixed in a lattice and cannot move.
Step 2: When molten, ions are free to move and carry charge.
Final Answer: Molten NaCl conducts electricity because ions are mobile.
Example
Explain why graphite conducts electricity but diamond does not, even though both are made of carbon.
▶️ Answer / Explanation
Step 1: In graphite, each carbon atom bonds to three others, leaving one free electron per atom — these electrons move freely between layers.
Step 2: In diamond, each carbon atom bonds to four others — no free electrons remain.
Final Answer: Graphite conducts electricity because of delocalized electrons, while diamond does not.
