IB MYP 4-5 Chemistry -Calculating relative formula mass- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Calculating relative formula mass- Study Notes
Key Concepts
- Calculating Relative Formula Mass (RFM)
- Empirical and Molecular Formula Calculations
Calculating Relative Formula Mass (RFM)
Calculating Relative Formula Mass (RFM)
The relative formula mass (RFM) of a compound is the sum of the relative atomic masses (Ar) of all the atoms shown in its chemical formula.
For molecular substances, the term relative molecular mass (Mr) is used, but both are calculated in the same way.
Symbol: \( \mathrm{M_r} \) or \( \mathrm{RFM} \)
Unit: None (it is a relative number — a ratio).
The RFM tells us how heavy one formula unit of a compound is compared to one atom of carbon-12 (the standard reference). It allows us to link the atomic world to measurable quantities like mass and moles.
Formula:
\( \mathrm{RFM = \sum (Ar \times number\ of\ atoms)} \)
- \( \mathrm{Ar} \) = relative atomic mass (from the periodic table)
- Multiply each element’s \( \mathrm{Ar} \) by how many atoms of that element are in the formula
- Add the results to find the total RFM
Step-by-Step Method to Calculate RFM![]()
- Write down the formula of the compound.
- List each element and the number of atoms of each.
- Find each element’s relative atomic mass (\( \mathrm{Ar} \)) from the periodic table.
- Multiply each \( \mathrm{Ar} \) by the number of atoms.
- Add all values to get the total RFM.
Common Atomic Masses (for Reference)
| Element | Symbol | Relative Atomic Mass (Ar) |
|---|---|---|
| Hydrogen | H | 1 |
| Carbon | C | 12 |
| Oxygen | O | 16 |
| Nitrogen | N | 14 |
| Sodium | Na | 23 |
| Chlorine | Cl | 35.5 |
Example
Find the relative formula mass of sodium chloride (\( \mathrm{NaCl} \)).
▶️ Answer / Explanation
Step 1: Elements: Na = 23, Cl = 35.5
Step 2: \( \mathrm{RFM = 23 + 35.5 = 58.5} \)
Final Answer: \( \mathrm{RFM(NaCl) = 58.5} \)
Example
Calculate the relative formula mass of calcium carbonate (\( \mathrm{CaCO_3} \)).
▶️ Answer / Explanation
Step 1: \( \mathrm{Ar(Ca) = 40,\ Ar(C) = 12,\ Ar(O) = 16} \)
Step 2: \( \mathrm{RFM = 40 + 12 + (16 \times 3)} \)
Step 3: \( \mathrm{RFM = 40 + 12 + 48 = 100} \)
Final Answer: \( \mathrm{RFM(CaCO_3) = 100} \)
Example
Calculate the relative molecular mass of ammonium sulfate, \( \mathrm{(NH_4)_2SO_4} \).
▶️ Answer / Explanation
Step 1: \( \mathrm{Ar(N) = 14,\ Ar(H) = 1,\ Ar(S) = 32,\ Ar(O) = 16} \)
Step 2: Number of atoms: \( \mathrm{(NH_4)_2SO_4 = 2N + 8H + 1S + 4O} \)
Step 3: \( \mathrm{RFM = (2 \times 14) + (8 \times 1) + 32 + (4 \times 16)} \)
Step 4: \( \mathrm{RFM = 28 + 8 + 32 + 64 = 132} \)
Final Answer: \( \mathrm{RFM((NH_4)_2SO_4) = 132} \)
Empirical and Molecular Formula Calculations
Empirical and Molecular Formula Calculations
The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula represents the actual number of atoms of each element in a molecule.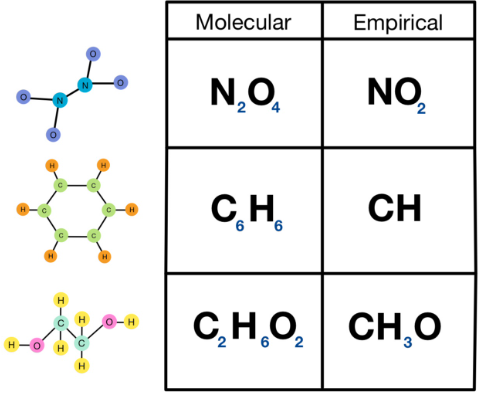
Empirical Formula
The empirical formula gives the simplest ratio of atoms of each element present in a compound.
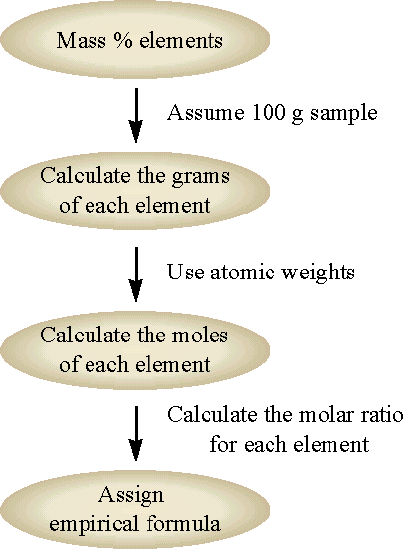
Steps to Determine Empirical Formula:
- Write the percentage composition or mass of each element.
- Divide each element’s mass by its atomic mass (Ar) to get moles.
- Divide all mole values by the smallest mole value to find the simplest whole-number ratio.
- Use the ratio to write the empirical formula.
Formula (conceptual): \( \mathrm{Ratio = \dfrac{m/Ar}{smallest\ (m/Ar)}} \)
Molecular Formula
The molecular formula shows the actual number of each type of atom in one molecule of a compound.
Relationship Between Empirical and Molecular Formulas:
\( \mathrm{Molecular\ Formula = (Empirical\ Formula) \times n} \)
where
\( \mathrm{n = \dfrac{Molecular\ Mass}{Empirical\ Formula\ Mass}} \)
- \( \mathrm{n} \) = whole number multiple
- \( \mathrm{Molecular\ Mass} \) = known molar mass from experiment
Example of Relationship:
If empirical formula = CH₂ (Mr = 14) and molecular mass = 28, then \( \mathrm{n = \dfrac{28}{14} = 2} \). So molecular formula = C₂H₄.
Summary Table: Empirical vs Molecular Formulas
| Property | Empirical Formula | Molecular Formula |
|---|---|---|
| Definition | Simplest ratio of atoms | Actual number of atoms |
| Formula | \( \mathrm{Ratio = \dfrac{m/Ar}{smallest\ (m/Ar)}} \) | \( \mathrm{Molecular = Empirical \times n} \) |
| Mass Relationship | Has lowest mass ratio | Multiple of empirical mass |
| Example | CH₂ | C₂H₄, C₃H₆ |
Example
A compound contains 40% carbon, 6.7% hydrogen, and 53.3% oxygen by mass. Find its empirical formula.
▶️ Answer / Explanation
Step 1: Assume 100 g of compound → C = 40 g, H = 6.7 g, O = 53.3 g.
Step 2: Divide by Ar:
- C: \( \mathrm{40 / 12 = 3.33} \)
- H: \( \mathrm{6.7 / 1 = 6.7} \)
- O: \( \mathrm{53.3 / 16 = 3.33} \)
Step 3: Divide all by smallest (3.33): C = 1, H = 2, O = 1
Final Answer: Empirical formula = \( \mathrm{CH_2O} \)
Example
The empirical formula of a compound is \( \mathrm{CH_2O} \). Its molar mass is 180 g/mol. Find its molecular formula.
▶️ Answer / Explanation
Step 1: Calculate empirical formula mass = \( \mathrm{12 + 2(1) + 16 = 30} \)
Step 2: \( \mathrm{n = \dfrac{180}{30} = 6} \)
Step 3: Multiply empirical formula by 6 → \( \mathrm{C_6H_{12}O_6} \)
Final Answer: Molecular formula = \( \mathrm{C_6H_{12}O_6} \) (glucose)
Example
On combustion, 0.1 mol of a hydrocarbon produces 0.4 mol of CO₂ and 0.5 mol of H₂O. Determine its empirical and molecular formula if the molecular mass is 58 g/mol.
▶️ Answer / Explanation
Step 1: Each CO₂ → 1 C atom, so 0.4 mol CO₂ → 0.4 mol C.
Each H₂O → 2 H atoms, so 0.5 mol H₂O → 1.0 mol H.
Step 2: Ratio of C : H = 0.4 : 1.0 = 2 : 5 (simplest ratio)
Step 3: Empirical formula = \( \mathrm{C_2H_5} \)
Step 4: Empirical mass = \( \mathrm{(2×12) + (5×1) = 29} \)
Step 5: \( \mathrm{n = \dfrac{58}{29} = 2} \)
Final Answer: Molecular formula = \( \mathrm{C_4H_{10}} \) (butane)
