IB MYP 4-5 Chemistry -Carboxylic acids- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Carboxylic acids- Study Notes
Key Concepts
- Carboxylic Acids (Structure, Properties, and Reactions)
Carboxylic Acids (Structure, Properties, and Reactions)
Carboxylic Acids (Structure, Properties, and Reactions)
Carboxylic acids are organic compounds that contain the carboxyl functional group (–COOH). They are weak acids that partially ionize in water to release hydrogen ions (\( \mathrm{H^+} \)). Their general formula is \( \mathrm{C_{n}H_{2n+1}COOH} \).
Structure and General Formula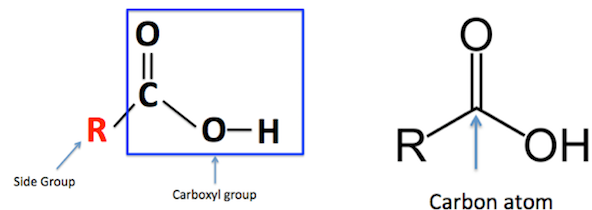
- Functional group: Carboxyl group (–COOH)
- General formula: \( \mathrm{C_{n}H_{2n+1}COOH} \)
- Homologous series: Carboxylic acids
Structural representation:
\( \mathrm{R–COOH} \), where R = alkyl group such as \( \mathrm{CH_3, C_2H_5, C_3H_7} \)
Examples:
- Methanoic acid (formic acid): \( \mathrm{HCOOH} \)
- Ethanoic acid (acetic acid): \( \mathrm{CH_3COOH} \)

- Propanoic acid: \( \mathrm{C_2H_5COOH} \)
Physical Properties
- Colorless liquids (lower members) with sharp, pungent odor.
- Soluble in water — due to hydrogen bonding with water molecules.
- Boiling points increase with molecular mass (due to strong intermolecular hydrogen bonding).
- Weakly acidic in nature (partially ionize in water).
Ionization in water:
\( \mathrm{CH_3COOH \rightleftharpoons CH_3COO^- + H^+} \)
Chemical Properties![]()
- (a) Reaction with Metals: React with active metals (like magnesium or zinc) to form a salt and hydrogen gas.
\( \mathrm{2CH_3COOH + Mg \rightarrow (CH_3COO)_2Mg + H_2} \)
- (b) Reaction with Bases (Neutralization): Form a salt and water when reacting with metal hydroxides.
\( \mathrm{CH_3COOH + NaOH \rightarrow CH_3COONa + H_2O} \)
- (c) Reaction with Carbonates / Bicarbonates: Produce a salt, water, and carbon dioxide.
\( \mathrm{2CH_3COOH + Na_2CO_3 \rightarrow 2CH_3COONa + H_2O + CO_2} \)
- (d) Oxidation: Alcohols can be oxidized to carboxylic acids using acidified potassium dichromate.
\( \mathrm{CH_3CH_2OH \xrightarrow{[O]} CH_3COOH + H_2O} \)

- (e) Esterification: React with alcohols in the presence of concentrated \( \mathrm{H_2SO_4} \) to form esters and water.
\( \mathrm{CH_3COOH + C_2H_5OH \xrightarrow{conc.\ H_2SO_4} CH_3COOC_2H_5 + H_2O} \)
Functional Group: –COOH (Carboxyl Group)
- Contains both a carbonyl (C=O) and a hydroxyl (–OH) group bonded to the same carbon atom.
- This combination allows it to release \( \mathrm{H^+} \), giving acidic properties.
- Carboxylic acids form hydrogen bonds easily — leading to high boiling points and solubility.
Uses of Carboxylic Acids
- Ethanoic acid is used in vinegar (about 4–8% solution).
- Used in making esters, plastics, and perfumes.
- Used in food preservation and as a cleaning agent.
- Higher acids are used in soap and detergent manufacture.
Comparison: Alcohols vs. Carboxylic Acids
| Property | Alcohols | Carboxylic Acids |
|---|---|---|
| Functional Group | –OH (Hydroxyl) | –COOH (Carboxyl) |
| Nature | Neutral | Acidic (weak acid) |
| Reaction with Metals | Slow or no reaction | Produces hydrogen gas |
| Ester Formation | React with acids | React with alcohols |
Example
Write the chemical equation for the neutralization of ethanoic acid with sodium hydroxide.
▶️ Answer / Explanation
Step 1: Ethanoic acid reacts with NaOH to form sodium ethanoate and water.
Step 2: \( \mathrm{CH_3COOH + NaOH \rightarrow CH_3COONa + H_2O} \)
Final Answer: Sodium ethanoate and water are formed in the neutralization reaction.
Example
Describe a chemical test to distinguish between ethanol and ethanoic acid.
▶️ Answer / Explanation
Step 1: Add sodium carbonate (\( \mathrm{Na_2CO_3} \)) to both samples.
Step 2: Effervescence (bubbling) indicates the release of \( \mathrm{CO_2} \) gas with ethanoic acid.
Step 3: No gas is produced with ethanol.
Final Answer: Effervescence occurs only with ethanoic acid, confirming its acidic nature.
Example
Calculate the volume of carbon dioxide produced when 10 g of ethanoic acid reacts completely with sodium carbonate.
▶️ Answer / Explanation
Step 1: Equation: \( \mathrm{2CH_3COOH + Na_2CO_3 \rightarrow 2CH_3COONa + H_2O + CO_2} \)
Step 2: Molar mass of \( \mathrm{CH_3COOH} = 60\ g/mol \)
Step 3: \( \mathrm{10\ g\ CH_3COOH = \dfrac{10}{60} = 0.1667\ mol} \)
Step 4: 2 mol acid → 1 mol \( \mathrm{CO_2} \), so \( 0.1667\ mol \) acid → \( 0.0833\ mol\ CO_2 \)
Step 5: Volume at STP = \( \mathrm{0.0833 \times 22.4 = 1.87\ L} \)
Final Answer: \( \mathrm{1.87\ L} \) of \( \mathrm{CO_2} \) is produced.

