IB MYP 4-5 Chemistry -Changes of state and energy transfer- Study Notes - New Syllabus
IB MYP 4-5 Chemistry -Changes of state and energy transfer- Study Notes
Key Concepts
- Changes of State
- Energy Transfer in Changes of State
Changes of State
Changes of State
A change of state is a physical change in which a substance transitions between solid, liquid, and gas without changing its chemical composition. The particles themselves remain the same, but their arrangement and movement change due to energy being absorbed or released.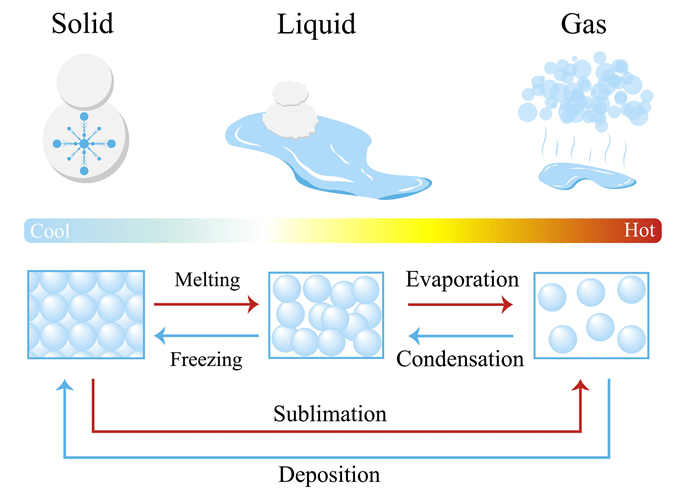
Energy and State Changes
The state of a substance depends on the balance between:
- Kinetic energy — energy of particle motion.
- Intermolecular forces — attractive forces holding particles together.
Changes of state occur when this balance shifts:
- When energy is absorbed: particles move faster, forces weaken → state changes to a higher energy level (solid → liquid → gas).
- When energy is released: particles slow down, forces strengthen → state changes to a lower energy level (gas → liquid → solid).
Common Changes of State
| Change of State | From → To | Energy Change | Description |
|---|---|---|---|
| Melting | Solid → Liquid | Energy absorbed (endothermic) | Particles gain energy, vibrate faster, and break free from fixed positions. |
| Freezing | Liquid → Solid | Energy released (exothermic) | Particles lose energy, move slower, and become locked into fixed positions. |
| Evaporation / Boiling | Liquid → Gas | Energy absorbed (endothermic) | Particles gain enough energy to escape into the gas phase. |
| Condensation | Gas → Liquid | Energy released (exothermic) | Particles lose energy and move closer together to form a liquid. |
| Sublimation | Solid → Gas | Energy absorbed (endothermic) | Particles gain enough energy to change directly from solid to gas. |
| Deposition | Gas → Solid | Energy released (exothermic) | Particles lose energy so rapidly that they form a solid without becoming liquid first. |
Key Points
- Changes of state are physical changes — no new substance is formed.
- Energy changes cause state transitions but not chemical changes.
- Temperature remains constant during a change of state until the entire transition is complete.
- The energy absorbed or released depends on the type of change and the amount of substance.
Example:
When ice melts, its temperature remains at 0°C until all of it becomes liquid water, even though heat is still being added. Explain why.
▶️ Answer / Explanation
Step 1: During melting, the temperature stays constant because added heat energy is not increasing the particles’ kinetic energy.
Step 2: Instead, the energy is used to overcome the strong forces between solid water molecules, allowing them to move freely as a liquid.
Step 3: Once all the ice has melted, any additional heat increases the temperature of the liquid water.
Final Answer: The temperature remains constant during melting because heat is used to weaken intermolecular forces rather than raise kinetic energy.
Example:
How much energy is required to completely melt 100 g of ice at 0°C? (Given: latent heat of fusion of ice = \( \mathrm{334\ J\ g^{-1}} \))
▶️ Answer / Explanation
Step 1: Use the equation \( \mathrm{Q = mL} \).
Step 2: \( \mathrm{Q = 100 \times 334 = 33400\ J} \).
Final Answer: \( \mathrm{33.4\ kJ} \) of energy is needed to melt 100 g of ice at 0°C.
Example :
On a humid day, droplets of water form on the outside of a cold glass of water. Explain this observation as a change of state.
▶️ Answer / Explanation
Step 1: The air around the glass contains water vapor (gas) at room temperature.
Step 2: When the warm, moist air contacts the cold surface, it cools below its condensation point.
Step 3: Water vapor changes from gas to liquid — a process called condensation.
Step 4: This change of state causes water droplets to appear on the glass surface.
Final Answer: The droplets form because water vapor in the air condenses into liquid water when cooled by the glass.
Energy Transfer in Changes of State
Energy Transfer in Changes of State
During a change of state, energy is transferred between a substance and its surroundings — either absorbed or released — in the form of heat. This transfer affects the energy of the particles (and their arrangement), but not the chemical identity of the substance.
Energy Transfer Overview
When a substance absorbs heat energy: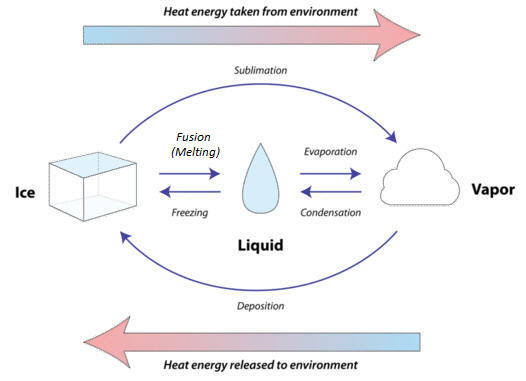
- Particles move faster and further apart.
- Intermolecular forces weaken.
- The substance changes to a higher-energy state (solid → liquid → gas).
When a substance releases heat energy:
- Particles move slower and come closer together.
- Intermolecular forces strengthen.
- The substance changes to a lower-energy state (gas → liquid → solid).
Temperature vs. Energy
During a change of state, the temperature of a substance remains constant even though energy is being transferred.
- The energy added or released is used to change the state, not the temperature.

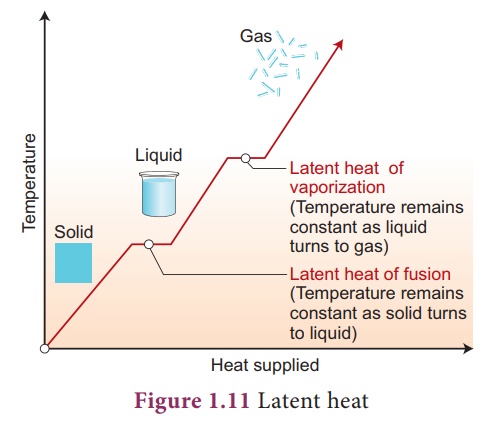
- This “hidden” energy is called the latent heat.
Latent Heat
The latent heat is the amount of heat energy absorbed or released by a substance during a change of state, without a change in temperature.
Formula:
\( \mathrm{Q = mL} \)
- \( \mathrm{Q} \): heat energy (Joules)
- \( \mathrm{m} \): mass of the substance (grams or kilograms)
- \( \mathrm{L} \): specific latent heat (J/g or J/kg)
Types of Latent Heat:
| Type | Process | Energy Change | Meaning |
|---|---|---|---|
| Latent Heat of Fusion | Melting / Freezing | Absorbed during melting, released during freezing | Energy needed to convert 1 g (or 1 kg) of solid into liquid at constant temperature. |
| Latent Heat of Vaporization | Evaporation / Condensation | Absorbed during boiling, released during condensation | Energy needed to convert 1 g (or 1 kg) of liquid into gas at constant temperature. |
Heating and Cooling Curves
Heating and cooling curves show how temperature changes when heat is added or removed from a substance. Flat regions indicate a change of state (temperature constant). Sloped regions indicate temperature change within a state.
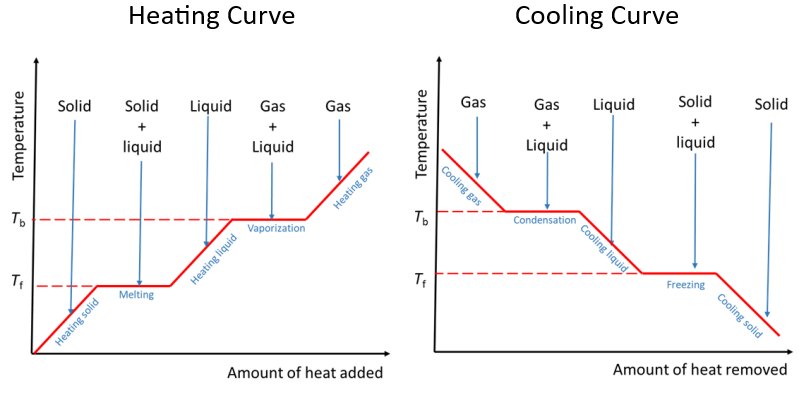
Explanation:
- Sloped parts → temperature increases (kinetic energy increases).
- Flat parts → energy used to change state (potential energy changes).
- The total energy of the system increases, but temperature doesn’t during phase change.
Key Relationships
- \( \mathrm{Q = mL} \) → Heat absorbed or released depends on mass and latent heat constant.
- Endothermic changes: melting, vaporization, sublimation (absorb energy).
- Exothermic changes: freezing, condensation, deposition (release energy).
- Temperature remains constant during state change; only the form of stored energy changes.
Example :
Why does the temperature of water stay at 100°C while it is boiling, even though heat is continuously supplied?
▶️ Answer / Explanation
Step 1: When water boils, added heat energy does not increase the kinetic energy of the particles.
Step 2: Instead, it is used to overcome the attractive forces between molecules so that they can separate and become gas.
Step 3: The temperature remains constant until all the liquid has turned into vapor.
Final Answer: The temperature stays constant because all added energy becomes latent heat used for vaporization, not for increasing temperature.
Example :
Calculate the energy required to change 200 g of water at 100°C to steam at 100°C. (Given: latent heat of vaporization \( \mathrm{L_v = 2260\ J\ g^{-1}} \))
▶️ Answer / Explanation
Step 1: Use the formula \( \mathrm{Q = mL_v} \).
Step 2: \( \mathrm{Q = 200 \times 2260 = 452000\ J} \).
Final Answer: \( \mathrm{4.52 \times 10^5\ J} \) (or 452 kJ) of energy is required to convert 200 g of water to steam at 100°C.
Example :
Explain why steam causes more severe burns than boiling water at the same temperature (100°C).
▶️ Answer / Explanation
Step 1: Steam and boiling water are both at 100°C, but steam contains extra energy — the latent heat of vaporization.
Step 2: When steam condenses on the skin, it releases this latent heat as it changes from gas to liquid.
Step 3: This transfer of energy adds to the heat already at 100°C, causing greater tissue damage.
Final Answer: Steam causes worse burns because it releases additional latent heat energy when condensing on the skin.